www diahome org Disclaimer The views and opinions





























- Slides: 48

www. diahome. org

Disclaimer The views and opinions expressed in the following Power. Point slides are those of the individual presenter and should not be attributed to Drug Information Association, Inc. (“DIA”), its directors, officers, employees, volunteers, members, chapters, councils, Special Interest Area Communities or affiliates, or any organization with which the presenter is employed or affiliated. These Power. Point slides are the intellectual property of the individual presenter and are protected under the copyright laws of the United States of America and other countries. Used by permission. All rights reserved. Drug Information Association, DIA and DIA logo are registered trademarks or trademarks of Drug Information Association Inc. All other trademarks are the property of their respective owners. www. diahome. org

Global Pharma Market: Performance and Outlook to 2013 Paul Crotty General Manager, IMS Health Canada www. diahome. org

Agenda • • Global Trends US Trends Canadian Trends Harbingers of Change www. diahome. org

Yesterday’s Model Will Not Deliver Tomorrow’s Growth • The availability of good low-cost alternatives for most common conditions combined with increasing payer control of prescribing drives irreversible change • R&D pipeline will not replace sales lost to generics - new products are fewer and smaller • Growth lies in new areas: specialist-driven therapies and emerging markets • Even in these areas, companies need to change their approach: – Earlier preparation for launch to gain payer acceptance and physician adoption – Realignment of commercial models – Leverage emerging markets to drive growth – Re-think R&D Surprisingly good performance in 2009 does nothing to alleviate the need for significant change Source: IMS Health Consulting 2009 www. diahome. org

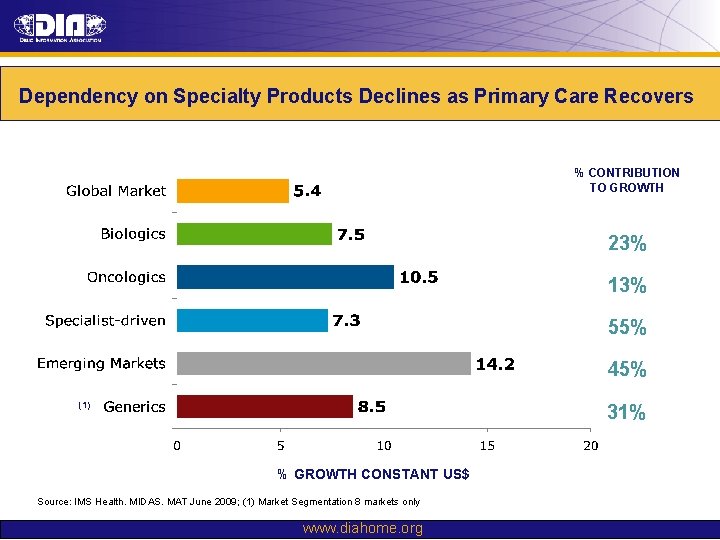
Dependency on Specialty Products Declines as Primary Care Recovers % CONTRIBUTION TO GROWTH 23% 13% 55% 45% (1) 31% % GROWTH CONSTANT US$ Source: IMS Health. MIDAS. MAT June 2009; (1) Market Segmentation 8 markets only www. diahome. org

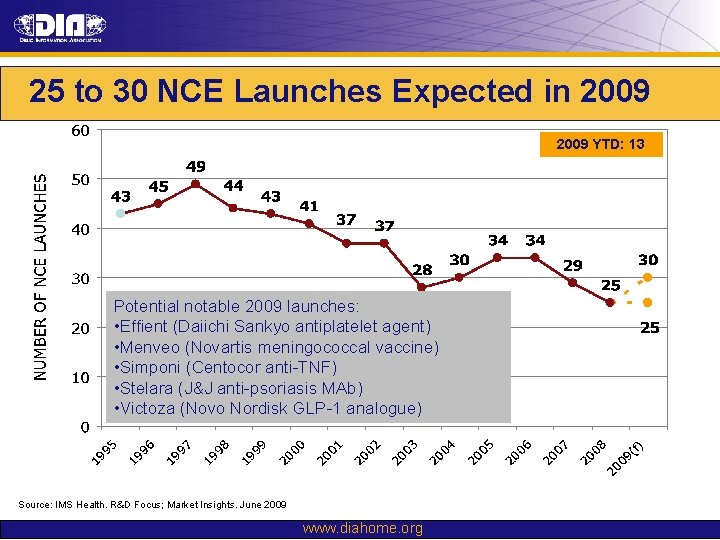
25 to 30 NCE Launches Expected in 2009 YTD: 13 Potential notable 2009 launches: • Effient (Daiichi Sankyo antiplatelet agent) • Menveo (Novartis meningococcal vaccine) • Simponi (Centocor anti-TNF) • Stelara (J&J anti-psoriasis MAb) • Victoza (Novo Nordisk GLP-1 analogue) Source: IMS Health. R&D Focus; Market Insights. June 2009 www. diahome. org

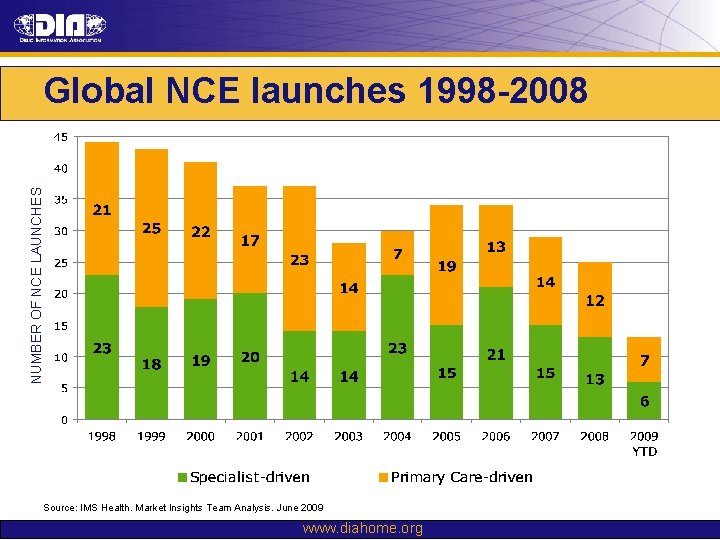
NUMBER OF NCE LAUNCHES Global NCE launches 1998 -2008 Source: IMS Health. Market Insights Team Analysis. June 2009 www. diahome. org

Global Outlook Through 2013 – Continuing Challenges for Most Major Markets • US new products will not fill the trough from the patent cliff despite more positive recent signs • Cost containment continues as a priority in major EU markets though access begins to open for innovative therapies, sometimes with risk-sharing agreements • South Korea, Turkey and Russia respond to budget pressure with significant reforms - outlook remains strong • Japan looking at pricing system overhaul - major implications for market performance and innovation …Global growth 4% to 7% www. diahome. org

Agenda • • Global Trends US Trends Canadian Trends Harbingers of Change www. diahome. org

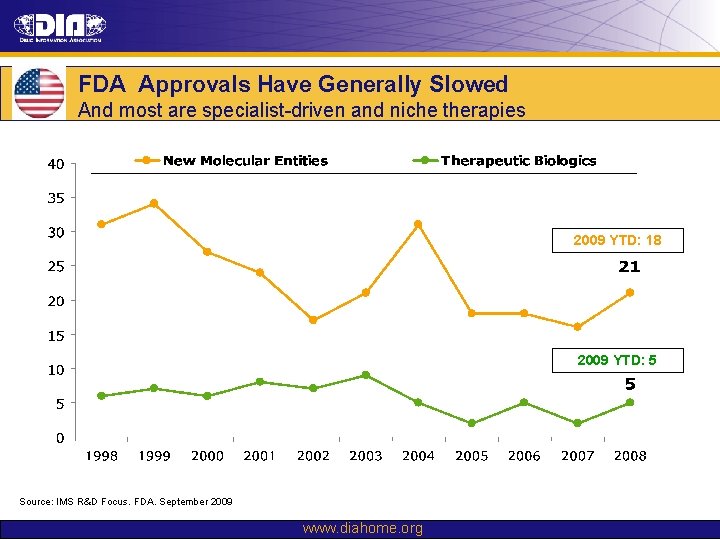
FDA Approvals Have Generally Slowed And most are specialist-driven and niche therapies 2009 YTD: 18 2009 YTD: 5 Source: IMS R&D Focus. FDA. September 2009 www. diahome. org

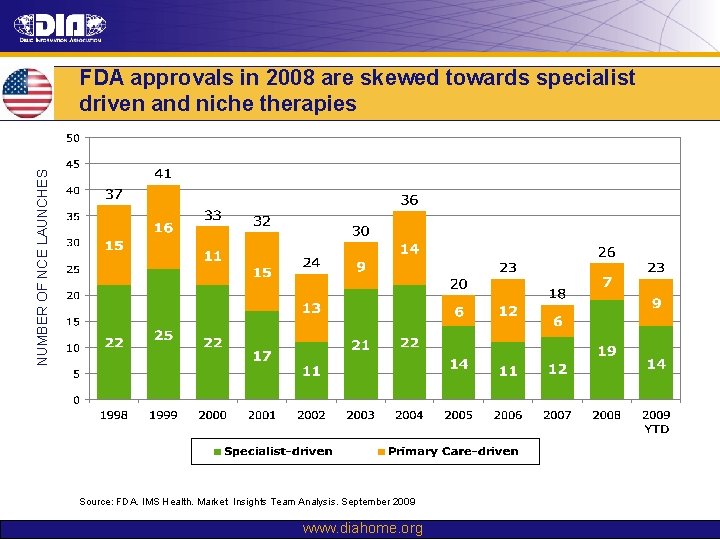
NUMBER OF NCE LAUNCHES FDA approvals in 2008 are skewed towards specialist driven and niche therapies Source: FDA. IMS Health. Market Insights Team Analysis. September 2009 www. diahome. org

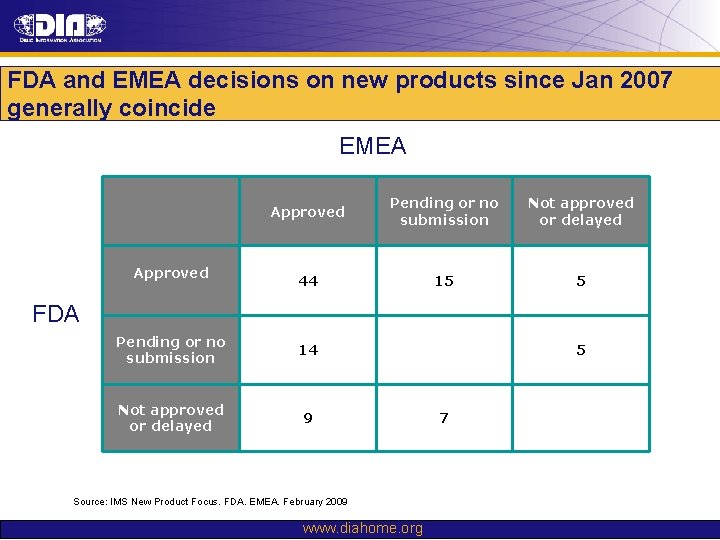
FDA and EMEA decisions on new products since Jan 2007 generally coincide EMEA Approved Pending or no submission Not approved or delayed 44 15 5 FDA Pending or no submission 14 Not approved or delayed 9 Source: IMS New Product Focus. FDA. EMEA. February 2009 www. diahome. org 5 7

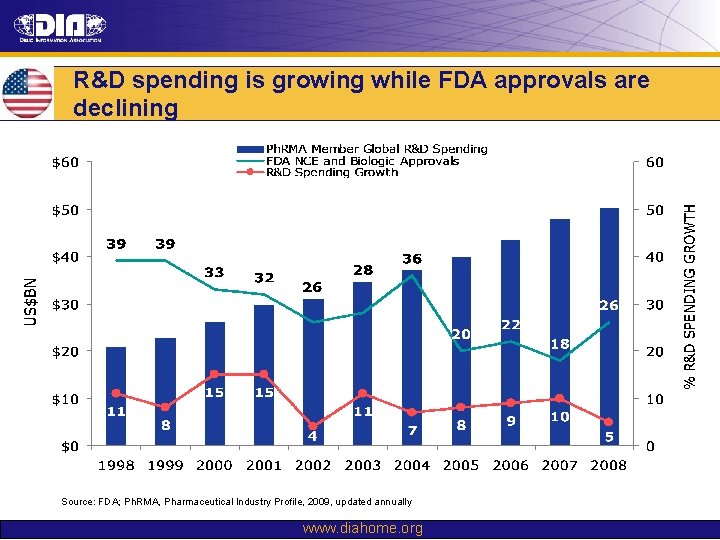
R&D spending is growing while FDA approvals are declining Source: FDA; Ph. RMA, Pharmaceutical Industry Profile, 2009, updated annually www. diahome. org

REMS in 2009 go hand in hand with major new product approvals • 4 of the 18 NCE approvals in 2009 to date require REMS (Risk Evaluation and Mitigation Strategies) • Major new products are arriving to the market with REMS that mandate Med. Guide, communication plans, and more: ? – Efient (prasugrel) – Simponi (golimumab) – Multaq (dronedarone) • 24 REMS issued Apr-Dec 2008 • 31 REMS issued Jan-Jul 2009 already and counting Source: IMS, Market Insights Research www. diahome. org

FDA is actively managing product safety through REMS, a FDAAA prerogative for the agency • Risk Evaluation and Mitigation Strategies are becoming prominent in all of FDA’s safety-related actions • Unlike standard post-marketing studies demanded by FDA with specific scope and patient populations, REMS are designed by companies and negotiated with FDA • REMS components can include: • Medication guide (for new or previously approved products) • Communication plan • Elements to assure safe use (healthcare providers and patients registries) • Implementation system ? • Medication guide-only REMS applied to multiple products in the following therapy classes: • Antiepileptics • TNF inhibitors • Erythropoietin stimulating agents • Fluoro-quinolones • First formal class-wide REMS for opioids and botulinum toxin products pending FDA implementation Source: FDA, IMS Market Insights Research www. diahome. org

FDA takes steps to improve its system for PMC design and monitoring • Deputy division director for safety - a position established under the agency's Safety First initiative - has assumed the postmarketing study coordinator job, and the safety regulatory manager will handle postmarketing study tracking; will manage safety and postmarketing study portfolio for their division • New policies and procedures are in place to "improve consistency in developing" post-market requirements and commitments and lead to "better designed studies and trials with effective and realistic time frames for initial completion”; improved tracking and reviewing, including standardizing policies and procedures across CDER review divisions and offices. ? • New postmarketing study DB implemented July 2009 will increase capacity for data capture, tracking and report generating Source: IMS, Market Insights Research www. diahome. org

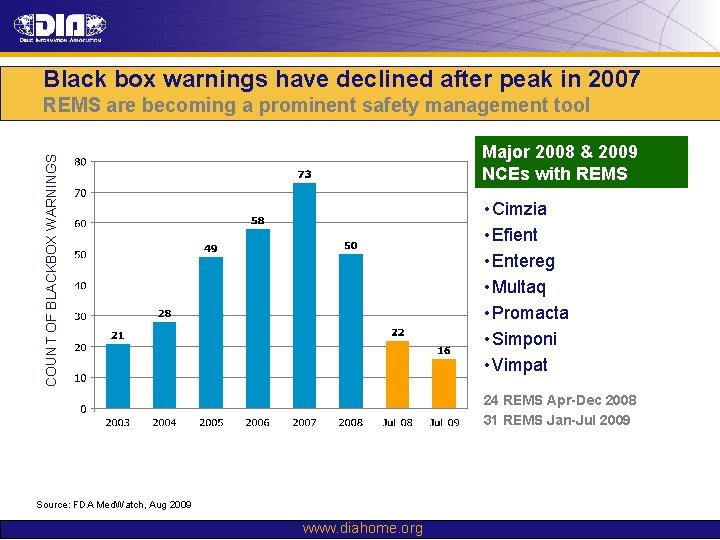
Black box warnings have declined after a peak in 2007 COUNT OF BLACKBOX WARNINGS Major boxed warnings in 2009 • Raptiva (withdrawn in EU and US) • Chantix, Wellbutrin for smoking cessation • Cimzia, Remicade for autoimmune diseases Source: FDA Med. Watch, Aug 2009 www. diahome. org

Black box warnings have declined after peak in 2007 REMS are becoming a prominent safety management tool COUNT OF BLACKBOX WARNINGS Major 2008 & 2009 NCEs with REMS • Cimzia • Efient • Entereg • Multaq • Promacta • Simponi • Vimpat 24 REMS Apr-Dec 2008 31 REMS Jan-Jul 2009 Source: FDA Med. Watch, Aug 2009 www. diahome. org

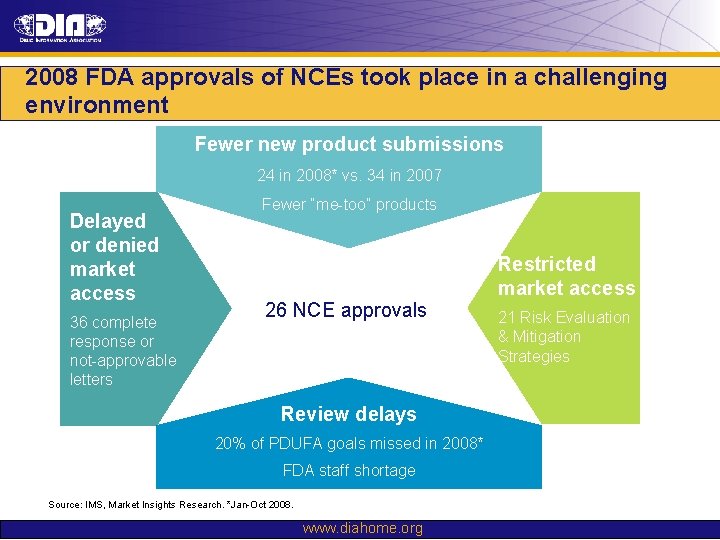
2008 FDA approvals of NCEs took place in a challenging environment Fewer new product submissions 24 in 2008* vs. 34 in 2007 Delayed or denied market access 36 complete response or not-approvable letters Fewer “me-too” products 26 NCE approvals Review delays 20% of PDUFA goals missed in 2008* FDA staff shortage Source: IMS, Market Insights Research. *Jan-Oct 2008. www. diahome. org Restricted market access 21 Risk Evaluation & Mitigation Strategies

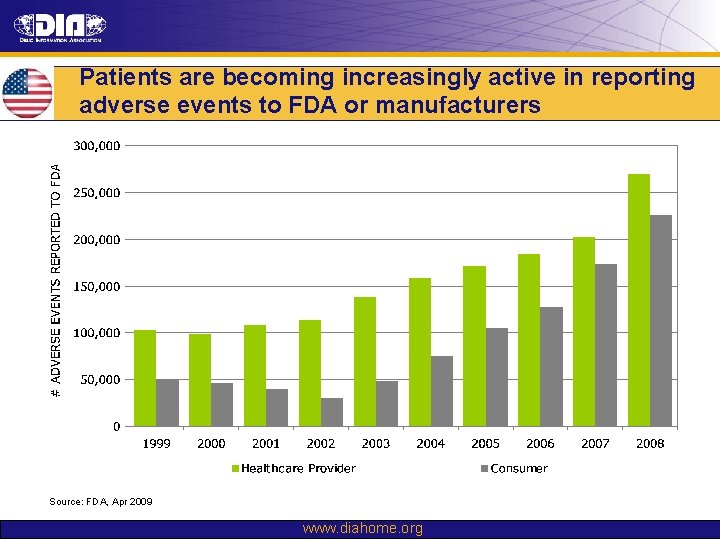
Patients are becoming increasingly active in reporting adverse events to FDA or manufacturers Source: FDA, Apr 2009 www. diahome. org

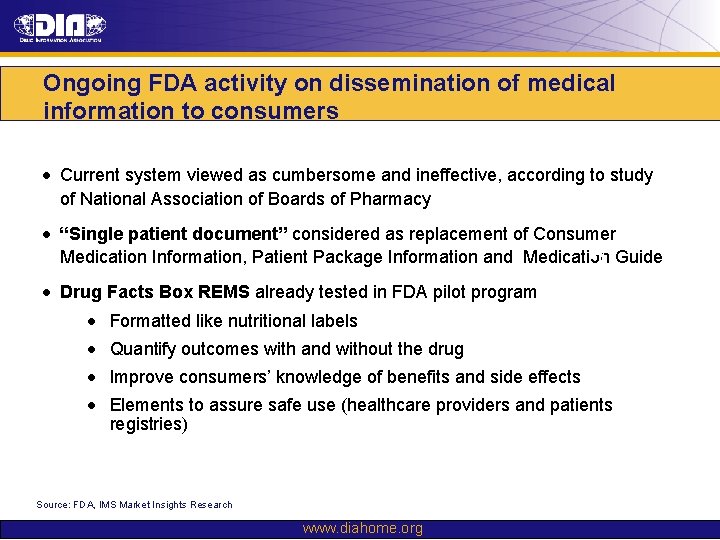
Ongoing FDA activity on dissemination of medical information to consumers • Current system viewed as cumbersome and ineffective, according to study of National Association of Boards of Pharmacy • “Single patient document” considered as replacement of Consumer Medication Information, Patient Package Information and Medication Guide • Drug Facts Box REMS already tested in FDA pilot program ? • Formatted like nutritional labels • Quantify outcomes with and without the drug • Improve consumers’ knowledge of benefits and side effects • Elements to assure safe use (healthcare providers and patients registries) Source: FDA, IMS Market Insights Research www. diahome. org

Agenda • • Global Trends US Trends Canadian Trends Harbingers of Change www. diahome. org

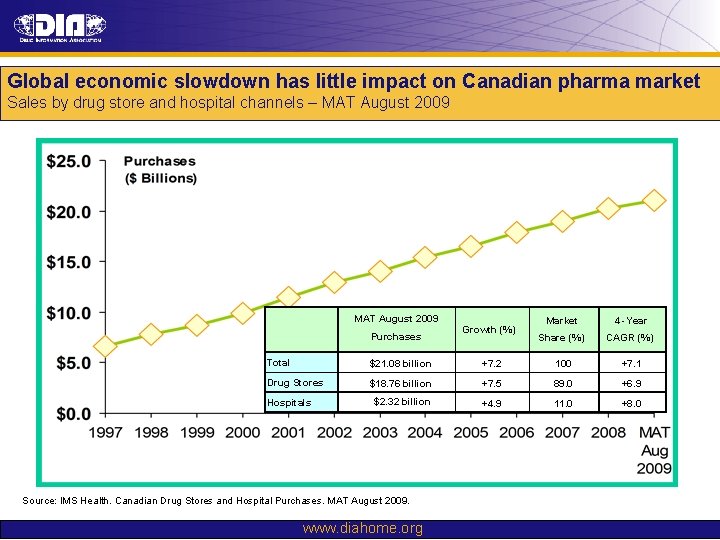
Global economic slowdown has little impact on Canadian pharma market Sales by drug store and hospital channels – MAT August 2009 MATJune August 2009 MAT 2007 Purchases Growth (%) Market 4 -Year Share (%) CAGR (%) Total $21. 08 billion +7. 2 100 +7. 1 Drug Stores $18. 76 billion +7. 5 89. 0 +6. 9 $2. 32 billion +4. 9 11. 0 +8. 0 Hospitals Source: IMS Health. Canadian Drug Stores and Hospital Purchases. MAT August 2009. www. diahome. org

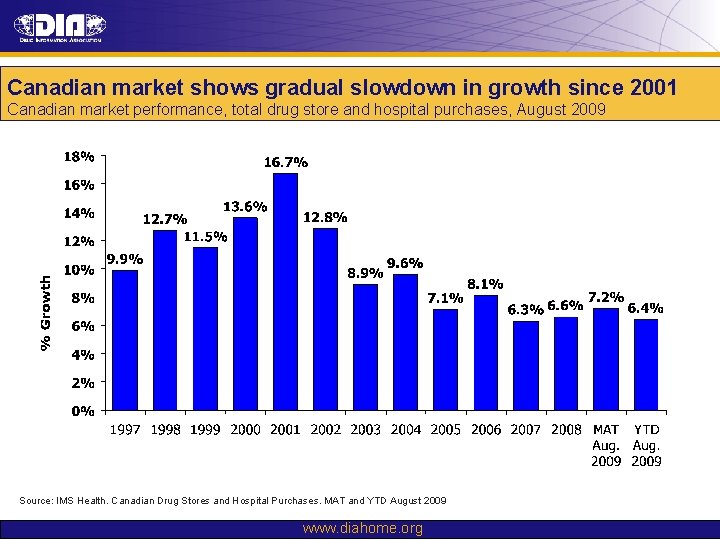
Canadian market shows gradual slowdown in growth since 2001 Canadian market performance, total drug store and hospital purchases, August 2009 Source: IMS Health. Canadian Drug Stores and Hospital Purchases. MAT and YTD August 2009 www. diahome. org

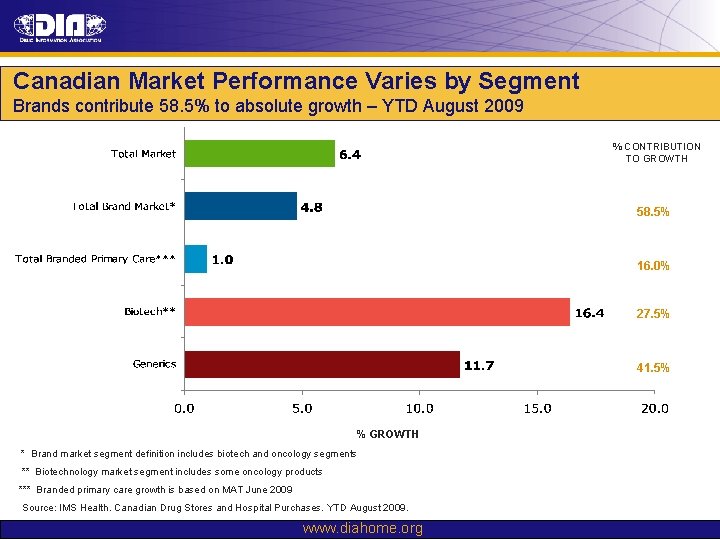
Canadian Market Performance Varies by Segment Brands contribute 58. 5% to absolute growth – YTD August 2009 % CONTRIBUTION TO GROWTH 58. 5% 16. 0% 27. 5% 41. 5% % GROWTH * Brand market segment definition includes biotech and oncology segments ** Biotechnology market segment includes some oncology products *** Branded primary care growth is based on MAT June 2009 Source: IMS Health. Canadian Drug Stores and Hospital Purchases. YTD August 2009. www. diahome. org

CDR Performance through October 9, 2009 Number of Submissions* 172 – Withdrawn/Suspended 11 – Active (under review) 13 – Completed submissions** 148 • Positive recommendations • Negative recommendations 70 (47%) 72 (49%) *Includes resubmissions, withdrawn submissions and Advisory Committee on Pharmaceuticals Requests for Advice **Includes 6 ACP Requests for Advice (No Final Recommendation issued) Source: IMS Health. Provincial Reimbursement Advisor (PRA Weekly), October 14, 2009. www. diahome. org

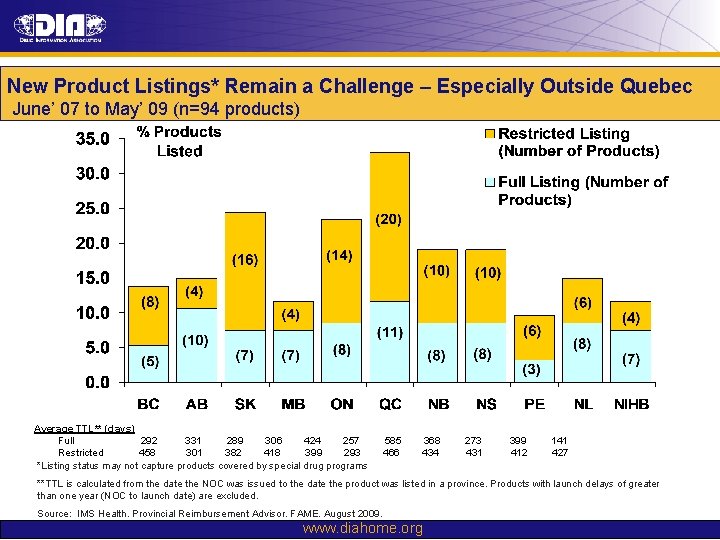
New Product Listings* Remain a Challenge – Especially Outside Quebec June’ 07 to May’ 09 (n=94 products) Average TTL** (days) Full 292 331 289 306 424 257 Restricted 458 301 382 418 399 293 *Listing status may not capture products covered by special drug programs 585 466 368 434 273 431 399 412 141 427 **TTL is calculated from the date the NOC was issued to the date the product was listed in a province. Products with launch delays of greater than one year (NOC to launch date) are excluded. Source: IMS Health. Provincial Reimbursement Advisor. FAME. August 2009. www. diahome. org

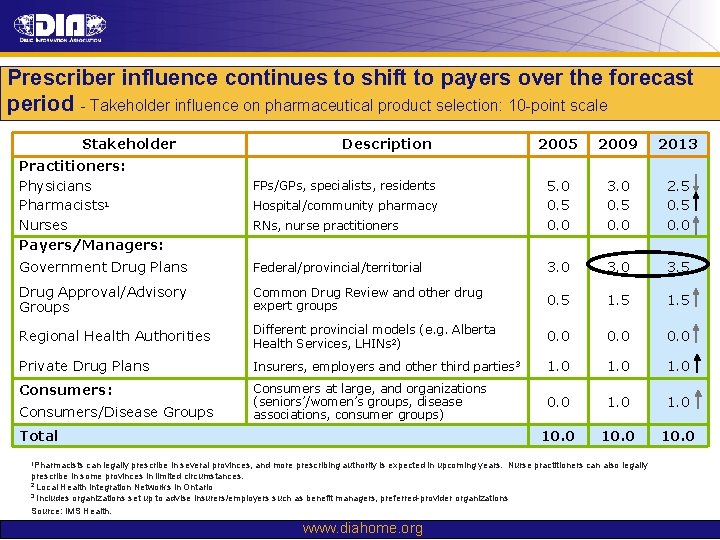
Prescriber influence continues to shift to payers over the forecast period - Takeholder influence on pharmaceutical product selection: 10 -point scale Stakeholder 2005 2009 2013 RNs, nurse practitioners 5. 0 0. 5 0. 0 3. 0 0. 5 0. 0 2. 5 0. 0 Government Drug Plans Federal/provincial/territorial 3. 0 3. 5 Drug Approval/Advisory Groups Common Drug Review and other drug expert groups 0. 5 1. 5 Regional Health Authorities Different provincial models (e. g. Alberta Health Services, LHINs 2) 0. 0 Private Drug Plans Insurers, employers and other third parties 3 1. 0 Consumers: Consumers at large, and organizations (seniors’/women’s groups, disease associations, consumer groups) 0. 0 10. 0 Practitioners: Physicians Pharmacists 1 Nurses Payers/Managers: Consumers/Disease Groups Description FPs/GPs, specialists, residents Hospital/community pharmacy Total 1 Pharmacists can legally prescribe in several provinces, and more prescribing authority is expected in upcoming years. Nurse practitioners can also legally prescribe in some provinces in limited circumstances. 2 Local Health Integration Networks in Ontario 3 Includes organizations set up to advise insurers/employers such as benefit managers, preferred-provider organizations Source: IMS Health. www. diahome. org

Harbingers of Change Transforming events in the pharmaceutical market Global Pharmaceutical Perspectives 13 December 2021 www. diahome. org

Harbingers of Change: Transforming Events in the Pharmaceutical Market Primum Non Nocere…to the Cardiovascular System: FDA Raises Approval Hurdles for Diabetes Medications Coloring Outside the Lines: R&D Companies Expand into Biosimilars A Compromised Immune System: Global Sourcing of API Overwhelms the FDA U. K. Gets Warm and Fuzzy: Permits “Top-Up” Payments for Drugs Not Covered by NHS Trimming the Smorgasbord: South Korea Mandates Economic Evaluation for National Reimbursement of Pharmaceuticals The Comeback Biz: Over-the-Counter Market Attractive to Pharmaceutical Manufacturers, Once Again All in One, One for All: The Polypill to Prevent Cardiovascular Disease among the Masses Giving Up on Godot: Shake Ups in R&D Source: IMS INTELLIGENCE. 360 (2008). http: //www. imshealth. com/portal/site/imshealth www. diahome. org

Trimming the Smorgasbord: South Korea Mandates Economic Evaluation for National Reimbursement of Pharmaceuticals THE HARBINGER In January 2008, new drugs became subject to a Health Technology Assessment prior to being covered for reimbursement by South Korea’s National Health Insurance System IMS INTELLIGENCE. 360 2008 www. diahome. org

South Korea follows in the footsteps of developed markets THE CHANGE South Korea became the first Asian country to use health economic data in determining reimbursement and pricing decisions Ministry of Health hopes to promote rational use in this healthcare paradise • 33 IMS INTELLIGENCE. 360 2008 www. diahome. org

Proving the value of medicines is becoming a global requirement THE IMPLICATIONS Conducting HEOR is increasingly a global exercise that can be made more efficient by adopting a core economic model to specific countries Companies will face greater price pressure in this growing market Other Asian markets (China, Japan, and Taiwan) are watching • 34 IMS INTELLIGENCE. 360 2008 www. diahome. org

Primum Non Nocere. . . to the Cardiovascular System: FDA Raises Approval Hurdles for Diabetes Medications THE HARBINGER Following the recommendation of an advisory panel, the FDA ruled that Type 2 Diabetes drugs in development must be shown to not increase cardiovascular risk—even when there are no red flags raised in Phase II and III trials. Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

FDA exercises greater caution THE CHANGE First time the FDA has been so prescriptive about what risks must be ruled out Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Companies must re-think their preapproval studies THE IMPLICATIONS Extreme regulatory caution seen in otherapeutic categories as well More robust, protracted trials the new reality, leading to higher costs and market entry delays Pre-approval studies must accommodate subpopulation analyses and be powered for adverse events, not just efficacy Developers should seek advice and guidance from FDA regularly Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Coloring Outside the Lines: R&D-Based Companies Expand into Biosimilars THE HARBINGER Merck & Co. announced that it was creating a new unit, Merck Bioventures, to produce new and follow-on biologics. Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Biosimilars hold promise of growth THE CHANGE Merck became the first major, pure-play R&D-based company to venture into biosimilars Other top-tier companies (Lilly, Pfizer, and Astra. Zeneca) also expressed their interest in the area Companies are open to exploring new avenues of growth, even if it means altering their fundamental business model Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Market will not mimic that of smallmolecule generics THE IMPLICATIONS Significant barriers to entry could limit competition, and uptake will be dependent on substitution laws The biosimilars market may be a good match for the skills inherent in an R&D-based company Development, production and marketing costs will limit the ability to compete on price Big pharma’s entrance into the market is a credibility boost for the sector Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Giving up on Godot THE HARBINGER During 2008, a number of “big pharma” companies shook up their research and development units with bold moves designed to spark innovation, improve productivity, and introduce a commercial focus into the scientific realm. In fact, of the top ten global pharmaceutical manufacturers, six announced major changes to their R&D models, and nine have made changes relating to the size, structure or focus of their R&D organizations. Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

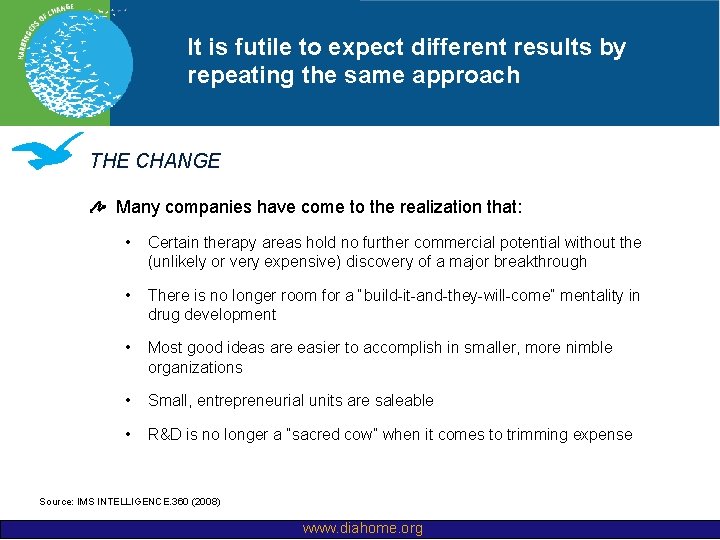
It is futile to expect different results by repeating the same approach THE CHANGE Many companies have come to the realization that: • Certain therapy areas hold no further commercial potential without the (unlikely or very expensive) discovery of a major breakthrough • There is no longer room for a “build-it-and-they-will-come” mentality in drug development • Most good ideas are easier to accomplish in smaller, more nimble organizations • Small, entrepreneurial units are saleable • R&D is no longer a “sacred cow” when it comes to trimming expense Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

R&D in big pharma and biotech meeting in the middle THE IMPLICATIONS Developers must be given incentives for not meeting payer criteria Shareholders need to be conditioned to the fact that R&D projects may have to take longer, cost more, and carry greater risk Large pharmaceutical companies must foster greater flexibility and openness within their research units There will be no place to hide for the researcher who is not suited to an entrepreneurial environment Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

R&D in big pharma and biotech meeting in the middle THE IMPLICATIONS Companies must be open to new ways of working—including forming partnerships at earlier phases of development Biotechnology companies must look beyond their big pharma customers to changing decision makers and their needs Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

A Compromised Immune System: Global Sourcing of API Overwhelms the FDA THE HARBINGER Violations in Good Manufacturing Practices (GMP) at an API producer in China claimed 149 lives and caused Baxter Healthcare to recall its heparin sodium injection. Later in the year, the FDA blocked more than 30 Ranbaxy products and APIs that originated in India because of GMP violations. Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Can the FDA be the “quality control” unit for the world? THE CHANGE China and India are now the primary producers of API used in the US The FDA is equipped to inspect only a smattering of the thousands of foreign plants each year Lawsuits in the heparin case are the first to involve Chinese manufacturing defects in drugs Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Some API suppliers “let the buyer beware” THE IMPLICATIONS US regulators’ efforts to inspect off-shore facilities will intensify Greater collaboration will develop between regulatory agencies Manufacturers must assume more of the burden for ensuring the safety of their supply chain and weigh savings against risks Source: IMS INTELLIGENCE. 360 (2008) www. diahome. org

Global Pharma Market: Performance and Outlook to 2013 Paul Crotty General Manager, IMS Health Canada www. diahome. org
 Views expressed disclaimer example
Views expressed disclaimer example All views expressed disclaimer
All views expressed disclaimer The views and opinions expressed
The views and opinions expressed The views and opinions expressed
The views and opinions expressed All opinions expressed disclaimer
All opinions expressed disclaimer Lindmat watch
Lindmat watch Disclaimer for opinions expressed
Disclaimer for opinions expressed The views expressed disclaimer
The views expressed disclaimer Economics systems
Economics systems Views expressed disclaimer
Views expressed disclaimer Disclaimer the views expressed
Disclaimer the views expressed Spoken poetry english
Spoken poetry english Summary of how to avoid foolish opinions
Summary of how to avoid foolish opinions Modal of suggestion
Modal of suggestion Asking and giving opinion example
Asking and giving opinion example Likes and opinions
Likes and opinions Expressing opinions and feelings
Expressing opinions and feelings Giving recommendations and opinions
Giving recommendations and opinions Structure of giving suggestion
Structure of giving suggestion Fact and opinion about pizza
Fact and opinion about pizza Fact statement example
Fact statement example Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi
Hát lên người ơi Các môn thể thao bắt đầu bằng tiếng bóng
Các môn thể thao bắt đầu bằng tiếng bóng Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên?
Thế nào là giọng cùng tên? Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế