Writing Your First Grant Sara Rockwell Ph D





















































































- Slides: 88

Writing Your First Grant Sara Rockwell, Ph. D Professor, Therapeutic Radiology and Pharmacology Associate Dean for Scientific Affairs Yale School of Medicine Postdoc Workshop, 1/11/11 1

Why write grants? • To get money to support the research you want to do • To support your career development • Current reality: institutional funds to support research and researchers at most medical schools is very, very limited • If you’re going to become a PI you will need to write successful grants • This is not easy (especially right now) 2

• Going from being a trainee performing research in a lab headed by another PI to being the PI of a project is a major transition. • Writing your first grant is the first step down this path to independence. • Many people begin by writing applications for fellowships and mentored awards or by writing small grants that they hold while still in the lab of a senior faculty member. • Even this is a big step new responsibilities new skills to learn 3

PI responsibilities - pre award • • • Securing appropriate institutional appointment Obtaining space and resources Signing Yale’s patent agreement Filing your COI form Taking the required PI training course Other compliance protocols and approvals (HIC, HIPAA, IACUC, Biosafety, etc) Completing the application materials Obtaining letters of support Adhering to institutional and agency deadlines Sending proposals to sponsors (some grants; 4 sometimes GCA must “press the button”)

PI responsibilities - post award • Conducting your research as proposed • Directly managing and administering your awards • Authorizing all direct cost expenditures of project funds • Approving all project related expenditures and cost transfers • Ensuring that all charges to an award are appropriate, including salary/wage charges for yourselves and others are charged to the award • Ensuring compliance with Human Subjects Protections; Animal Care and Use; Conflict of Interest disclosures; and other safety and responsible conduct of research regulations and guidance • Reporting scientific progress to grantmaker as required 5

Administration is a major part of the PI’s responsibility and effort PIs on average spend more than 40% of their time on administrative issues directly related to their research grants • Completing training and requirements (PI training, COI, IRB, RCR) • Writing related research protocols (IRB, IACUC Biosafety, etc. ); ensuring compliance • Assembling team; ensuring their training • Continuing reviews; reports during project • Managing personnel • Managing finances 6

Your are going to need help Fortunately, there are people who can and will help you • • Department Business Office Grant and Contract Administration Sponsor Others 7

Departmental Business Office • The business office provides administrative support services to the PI • Business office staff are the ‘Go to’ persons who will: § Assist with proposal preparation § Monitor awards and execute authorized transactions § Keep the PI abreast of policy and sponsor requirements and changes in these requirements § Develop appropriate local business processes for the administration of sponsored projects § Provide reports to the PI on award status 8

Grant and Contract Administration • Communicates changes in policy • Reviews applications for compliance • Negotiates terms and conditions against standards • Primary contact with funding agency both pre and post award • Partners with financial offices upon award to set up and manage award 9

Other offices that can help • • • Office of Research Administration Office of Strategic Research Initiatives HRPP Office (HIC/IRB Office) HIPPA Office IACUC Office Safety Office Conflict of Interest Office Faculty Office Dean’s Office YCCI Development Office 10

The Old World: The NIH Mailroom The new world: ERA 11

Electronic Research Administration (ERA) • ERA has made the grants world both easier and more difficult § § § Standardized formats (in theory) Complicated routing structures More non-standard funding mechanisms, RFAs, RFPs More PI responsibilities More non-standard submission dates More changes, made more rapidly (and less carefully) • Leave extra time for electronic submissions § The systems often crash on deadline dates! § The old 2 -day “window” to make corrections on NIH applications is gone as of January 25, 2011. • Errors often occur during uploading. Check every page of every file to be sure it’s there, and still legible, correct, complete, and the right length. • Check your application’s progress! 12

Right now there are continuing changes at Yale and beyond • Continuing changes in funding mechanisms, policies, application forms, submission procedures, submission deadlines, review criteria and review procedures • Info. Ed (new internal grant writing and submission system, coming all too soon) • Pub. Med • Clinical trials. gov • Stem Cell Research • ARRA requirements • Be sure you have the latest information 13

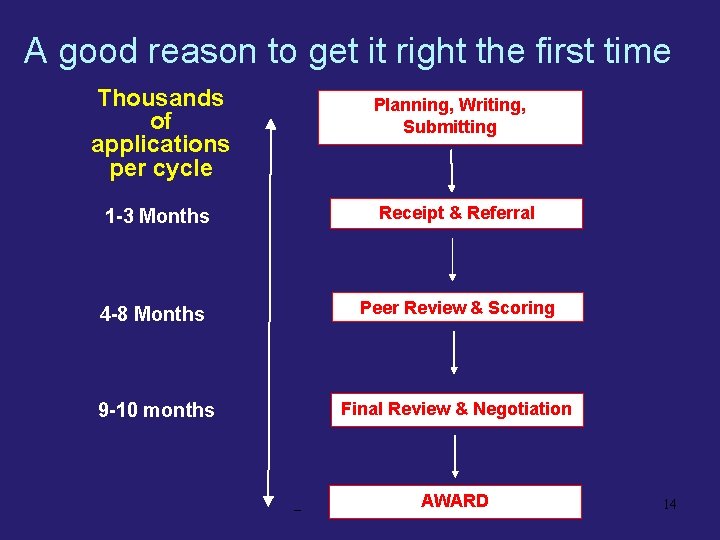
A good reason to get it right the first time Thousands of applications per cycle Planning, Writing, Submitting 1 -3 Months Receipt & Referral 4 -8 Months Peer Review & Scoring 9 -10 months Final Review & Negotiation AWARD 14

The Writing Process • When to start? • At least three months in advance § Longer for new project § Longer for complex project • Don’t assume that a renewal will be automatic or easy § Competitive renewals are as hard to get as new grants § Sometimes harder, if “new investigator” advantage is lost 15

Research Grants and Career Development Awards • Research grant: focus is on the merit of the project • Career development award: focus is on the potential of the applicant § Different foci § Different requirements § Even when you use the same research project for both kinds of grants, you will write them very differently 16

You can (and probably should) apply for more than one grant for your project • “Pay line” is often less than 20% • Same project to different agencies • Research project + career development award • Acknowledge overlap in “other support” sheets • If they are all funded § Celebrate § Decide which award (or sometimes awards) to accept and which to decline (GCA can help) 17

How to find funding sources • • Talk with colleagues Talk with business office/chair Talk to Melanie Smith in ORA/SRI Search databases on GCA website Utilize alert services Professional society websites YSM and Yale bulletin boards, list serves, announcements, etc. • Explore your options broadly! 18

Limited competitions • • “Scholars Awards” Usually career development awards Often limited to a narrow subject area Often limited to junior faculty Some open to or limited to postdocs Often very prestigious – big career boost Institution may be allowed to nominate only 1 or 2 two candidates • Internal competition to select Yale’s nominee(s) • Listed on GCA website • Melanie Smith can provide information 19

Internal competitions • • Grants through programs at Yale Often limited to Yale researchers Generally very focused Sometimes limited to new investigators Some Postdoctoral Fellowships Some Career Development Awards Some research grants § Generally small § Often for pilot studies • Can be very valuable § Get preliminary data § Establish that you can be an independent PI § Establish your track record of success as a PI 20

A few examples • Brown Coxe Fellowships • Anna Fuller Fellowships • Cancer Center Postdoctoral Fellowships and Pilot Projects • YCCI (CTSA) Scholars Program and CTSA Pilot Projects • Skin Center Pilots • Hematology Pilots 21

Explore all opportunities • Federal Agencies § NIH, NSF, DOD, DOE, NASA, others • Small Federal grant programs (e. g. R 03) • Non Federal sponsors § Foundations § Industry § State and local organizations • • Voluntary Health Agencies Professional Societies Think and look very broadly No grant is “too small” for your first grant 22

Responding to an RFA or RFP • Some Requests for Applications and Requests for Proposals are great opportunities; others are not worth the effort • Talk to the contact person • Find out more about the request, the intent, the criteria for funding, and the scope • Find out about the review process – who will be reviewing your grant? • Is money set aside? • How many projects will they fund? 23

Where to start: Gather information about grant and grantmaker • • • Grantmaker’s areas of interest Grantmaker’s policies Amount and duration of funding Deadlines Instructions Application forms Procedures used to review grants Time until funding Probability of funding 24

Gather the information needed to plan and develop your application • • • Literature related to project Resources needed for project Techniques needed Possible collaborators and mentors People who can be asked to write letters Cost and budget information • Make a list of everything you need to do before submitting the grant 25

Some critical things to think about before you begin to write • Are you eligible? § Position title § Time in position § Citizenship • Do you have the resources you need? § Skills § Equipment, facilities § Support from your department, institution • If not, can you get them? • What scope of project can you perform with your resources and time? • Don’t waste your time preparing grant applications that can’t fly 26

Things to keep in mind • If this project is successful, why will the world be a better place? • How does this project relate to the interests of the funding agency? • Why is your research strategy the right one for use in this project? • Use these to target the proposal to the appropriate funding agency and to sell the grant to the reviewers and program people 27

Remember: Reviewing and Funding are separate actions by different groups • Study Sections / Review Panels § Review applications for scientific merit § Prioritize by scientific merit • Program Officers fund projects § § Consider the scientific reviews and rankings Also consider priorities of program Consider balance of their portfolio May “reach” for applications in areas they feel are critical or under funded § May skip applications of low interest to their program 28

When you have questions • Talk to your Business Office • Talk to your GCA representative • Contact the grantmaker § Program people (scientists) § Administrators • Talk to experienced investigators in your field of research § Senior investigators § Young investigators, a couple years ahead of you § Successful applicants for same grant 29

Writing the application • Formats and contents of applications vary dramatically for different agencies § Read the instructions § Follow them to the letter § You will need to alter focus for different agencies and grant opportunities § You will need to alter scope of work to match money and time available § You will need to re-write to fit length, format • One size does not fit all…or even most 30

But you already knew that! 31

Watch for special requirements in career development applications • Letters of recommendation • Statement of long range career goals • Statement describing the relationship between this project and your long range professional goals • Plans for course work § Responsible Conduct of Research § Statistics § Courses related to the research • Interviews for finalists • Agreement to attend or speak at meetings 32

Important parts of the application • • Cover sheet Abstract or abstracts Administrative elements Assurances Biosketches or CVs Scientific sections Letters (sometimes) Appendices (sometimes) 33

The cover sheet • Specific to agency and grant type • Will have very specific format and instructions • May require very specific (and sometimes very bizarre) information § Some you will not know § Go to your Business Office and the Grants and Contracts website and for help • May require signatures and assurances • Must be complete and accurate 34

Assurances • Don’t panic at the terrifying list of required assurances • Many already have been handled by the institution • You will need to handle some § § § § § Human subjects protection (HIC; HIPPA) Animal welfare (IACUC) Biosafety, Radiation, Environmental Health (OEHS) Conflict of Interest and Commitment Patent assignment Export Controls Responsible Conduct of Research (RCR) Scientific Misconduct Data sharing /Data management Mentoring 35

Picking a title for your project • Sounds trivial…but isn’t • Length may be quite limited • Be informative: § Titles may be used to assign grants to review committees and to individual reviewers § Titles may be sent to reviewers to allow them to the select grants they want to review • Should be intelligible to non-specialists • Don’t use jargon • Don’t get cute 36

Abstract • Draft first; then edit/rewrite when your application is almost done • May be the most important part of application § Used to assign committees and reviewers § Reviewers may use to select grants for review § Read by all reviewers on panel • The abstract should summarize your project, describe its importance, and make the reader excited about reading the application and funding the project 37

Lay abstract • Many agencies require lay abstracts • Very important § There may be non-scientists on the review panel § Foundations give these abstracts to their donors • • • Can be difficult to write Write it for an intelligent non-scientist Describe project in non-technical terms Emphasize importance and relevance Ask some non-scientists to read and critique your draft 38

CV or Biosketch • Very important element of any grant • Absolutely critical for fellowships and career development awards • Primary reviewers will examine this very carefully • Other reviewers will look at it before and during meeting - especially if there are questions or problems • Different from your resume and from your full academic CV • Focus tightly on information relevant to your research career and your project 39

Preparing the Biosketch or CV • Format varies with grantmaker • Look forms and detailed instructions § § Follow them exactly Do not alter order from that specified Proofread, proofread Do not exceed the allowed length • Sections usually include § § § Current position Education Personal Statement (NIH: specific to application) Professional Experience Honors and Awards Publications , 40

Biosketch: Current Position • Be sure your Current Position on the CV matches that on cover and elsewhere • Use your official University title • Promotion in progress? § List effective date § List only positions that have been offered and accepted in writing § You may be asked to provide documentation § If application includes letter from the Chair, Dean or Mentor be sure that letter mentions the pending promotion 41

Education and Experience • Generally: start with college • Include areas of study and degrees earned • Non-degree programs and education may warrant inclusion • Include all graduate and postdoctoral training and research § Broad outline: start and end dates, institution, city, state, country, mentor § Don’t give details of project or activities § NIH: education goes in boxes, wanting less information • Chronological, but watch order 42

Experience and Awards • Experience may go beyond your primary job appointment, if it is relevant to application § Secondary appointments § Advisory boards § Some other experience and activities (e. g. teaching, certain community activities) • Avoid unexplained gaps • Awards and honors § § § Select with care Begin with college Do not include trivial awards Awards relevant to professional career Describe award if implications may be unclear to an outside observer 43

Publications • Follow instructions format and content very carefully – great variation between grantmakers • Reviewers will look at § Number of publications § Evidence of a good trajectory of publication § Quality of publications Peer reviewed journals? Quality, impact of journals? Full article or brief notes and case reports? § Your position as author § How many authors? § Who are the other authors? § Relevance to proposed research • Warning: Negotiate your authorships carefully 44

Publications • Usually allowed § Papers published in peer reviewed journals § Papers in press (this means the paper has been § § § accepted for publication) Books Book chapters, full papers in peer reviewed proceedings, review articles (may be separate) Abstracts - maybe. Specify and list separately • Do not include § Papers in preparation § Papers submitted but not yet accepted Plan ahead - submit early Can sometimes send new papers after they have been accepted 45

Publications • Look for restrictions on the number of publications § New NIH Biosketch format specifies a maximum of 15 recent, relevant publications § NSF wants 5 publications relevant to the project § Select with care! • Check formatting requirements (e. g. NIHMS or PMC # for NIH Biosketches) • Some agencies also ask for your total number of publications • If you have more publications than allowed consider including an opening statement such as “Selected from a total of 195 publications” • If you have only fewer than the allowed number of publications, include them all 46

Budget • Format and required information vary dramatically • Some agencies specify a fixed budget and define how you must spend it. • Some want budget details • Some want none • Give them what they want • Use the forms or follow the format given in the instructions • Check agency guidelines: what costs are allowable and what are not? § You won’t get money for unallowable items § Watch how Indirect Costs (Facilities and Administrative Costs) are handled. 47

Developing your numbers • Even if the agency doesn’t want details, work up a budget so you know what you can do with the funds available • Use real numbers § Real salaries and fringes § Real costs of supplies, animal care, etc § Include everything you will need • Extrapolate costs to actual start date of grant • Don’t “low ball” • Don’t forget the F&A costs 48

Future years • Extrapolate from first year budget • Consider changes in project over time; the science and the budget should always correspond • Project future salaries as accurately as possible § Include expected raises and promotions § Business office can help here • Increase other costs to allow for inflation 49

PROBLEM: Constant budgets • Some agencies fund grants at a constant level for future years § NIH modular grants § Grants with total budget set by agency • May allow carryover of funds • Remember to plan for raises and inflation in deciding how much money you request in the first year • HINT: for a 3 year grant use second year cost estimates (not current year values or first year cost estimates) to develop the budget for the project 50

Budget Justification • Format and detail required vary greatly for different applications • Follow instructions carefully • Always justify your costs in terms of the science of the project • Will be examined by study section members (scientists) during their review • Will be examined later by business people and accountants 51

Time and effort is examined closely by the reviewers • Does it match the scientific activities you have described? • Do you have enough time from the people who are essential to the project? • Do you have all the skills you need? • Do you have enough technical support? • A very common problem with grants from young investigators is that the project described cannot possibly be performed with the resources available. 52

Expectations on time/effort • Percent Salary = Percent Effort § If not, you must justify the difference § Effort generally is not allowed without salary support • You cannot have more than 100% professional effort § All Yale assignments § All external professional activities • Watch efforts in application carefully. If you are funded: § You may be held to the promises you’ve made § You will be asked to document the efforts of those on the grant 53

Resources and Environment • Space • Equipment • Core facilities § § Departmental School of Medicine University External • Expertise, equipment and facilities available through your co-investigators • External resources to be used • Watch grantmaker instructions § NIH: description must now be specific to the project § Watch for new requirements for Early Stage Investigators and Career Development Awards 54

Resources and Environment • For critical resources and expertise that you don’t have yourself, you may need to get letters of collaboration • You have an advantage by being at Yale § Many talented scientists, willing to share their expertise and resources § Great core facilities E. g. Keck center, West Campus cores Internationally known Available on fee for service basis If you’re going to use them, say so in the grant and budget justification 55

Scientific Sections • Format varies with sponsor • Follow instructions exactly • Conform to required length § Can be shorter § Can never be longer • Don’t try to get around length limits by using tiny fonts, small margins or appendices § Many agencies reject such grants without review § Even if they don’t, the reviewers are usually ruthless and unsympathetic § Everyone else has the same space limit 56

Scientific sections of an NIH application (revised in May 2010) • Specific Aims • Research Strategy § Significance § Innovation § Impact • Literature Cited • Appendices - sometimes 57

Specific Aims • Short paragraph describing overarching goal of project • Brief list of specific things you plan to accomplish § 3 - 5 Aims § May have sub-aims • Length 1/2 to 1 page • Broad overview of goals, hypotheses to be tested and approaches to be used, in telegraphic form 58

Specific Aims (from NIH instructions) • State concisely the goals of the proposed research and summarize the expected outcome(s), including the impact that the results of the proposed research will exert on the research field(s) involved. • List succinctly the specific objectives of the research proposed, e. g. , to test a stated hypothesis, create a novel design, solve a specific problem, challenge an existing paradigm or clinical practice, address a critical barrier to progress in the field, or develop new technology. 59

Significance (from NIH Instructions) • Explain the importance of the problem or critical barrier to progress in the field that the proposed project addresses. • Explain how the proposed project will improve scientific knowledge, technical capability, and/or clinical practice in one or more broad fields. • Describe how the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field will be changed if the proposed aims are achieved. 60

Innovation (from NIH Instructions) • Explain how the application challenges and seeks to shift current research or clinical practice paradigms. • Describe any novel theoretical concepts, approaches or methodologies, instrumentation or interventions to be developed or used, and any advantage over existing methodologies, instrumentation, or interventions. • Explain any refinements, improvements, or new applications of theoretical concepts, approaches or methodologies, instrumentation, or interventions. 61

Approach (from NIH instructions) • Describe the overall strategy, methodology, and analyses to be used to accomplish the specific aims of the project. …include how the data will be collected, analyzed, and interpreted as well as any resource sharing plans as appropriate. • Discuss potential problems, alternative strategies, and benchmarks for success anticipated to achieve the aims. 62

Approach (continued) • If the project is in the early stages of development, describe any strategy to establish feasibility, and address the management of any high risk aspects of the proposed work. • Point out any procedures, situations, or materials that may be hazardous to personnel and precautions to be exercised. 63

Organizing the Research Strategy • If there are multiple Specific Aims, you may address Significance, Innovation and Approach for each Specific Aim individually, or you may address Significance, Innovation and Approach for all of the Specific Aims collectively. • Preliminary studies – used to be a separate section of the old NIH application. Now they are to be included in the Research Strategy, within the Significance, Innovation, and Approach sections. Despite this change, preliminary studies are still very important. 64

The reviewers will examine your preliminary data critically • To evaluate the basis and feasibility of the project • To predict the chance of success • To evaluate your § § § Ability to develop and test hypotheses Ability to design rigorous experiments Expertise with experimental techniques Expertise in analysis of data Rigor in interpreting the data Ability to present findings clearly and effectively • Sloppiness here is absolutely fatal 65

Preliminary Studies • Your own preliminary data § § § Present your data carefully and clearly Use high quality graphs, photos, and tables Show, discuss appropriate controls Analyze appropriately Use appropriate statistics Interpret your findings carefully and critically; acknowledge limitations of techniques and data • Can include closely related work by others 66

In describing the Research Strategy be sure to give the “big picture” • The Approach is not just methods • Outline your experimental approach § Base on specific aims - restate aims and describe flow of experiments under each aim § Develop logic of project § Describe timeline, sequence of experiments § Describe potential pitfalls and what you will do if they occur • Point out significance and innovation • Talk about clinical relevance (for those grantmakers with clinical interests) 67

When describing methodology • Cite appropriate references • Establish your expertise with the techniques to be used § give citations to your work using the techniques § provide accurate discussion of techniques and of their strength and limitations § give methodology details where critical (especially if unpublished or unique) § describe alternatives you will use if a technique is inadequate or the results are inconclusive § Don’t forget data analysis and statistical analyses • The new NIH format was supposed to decrease emphasis on methodologic details, but many reviewers still seem to want them. 68

For techniques that are new for you Tell how you will obtain expertise • Collaborator § Yale include as investigator § Outside consultant: biosketch, letter, subcontract: agreement between institutions • Someone who will teach you § Letter § Biosketch • Use a core facility • Take a course 69

Literature Cited • Follow required format exactly • Be complete, but not silly • Be accurate § read entire article carefully § cite accurately • Remember: some reviewers will probably be people reading and publishing in this area • Include your own work but also cite others, including competitors • Don’t ignore literature you don’t like, instead cite and discuss it 70 • Be objective

Appendices • Follow instructions carefully § Some applications have mandatory appendices § Some do not allow appendices § Some limit appendices • Possible appendices § § § NIH: animals, human subjects, sharing resources NSF: mentoring, data management / sharing Letters of collaboration Letters of recommendation Papers Manuscripts in press 71

Warnings about appendices • Sometimes only the primary reviewers have them • Some reviewers never look at them • Do not try to use them to circumvent page limitations • Do not use them for critical information; mention ever critical point in the body of the application • They may be separate from the main application - label them clearly 72

Letters of Recommendation • Sometimes required • Examined with great care • Letters should discuss your past and current work and your long range potential in your chosen profession • Select sponsors carefully § Professional references, not personal references § Ideally, include thesis advisor, postdoctoral advisor, and someone who knows your present work § Select people who know your work, are reasonably senior, and know how to write good letters 73

When requesting letters • Provide instructions from the grantmaker. § § Specific information may be requested Specific format may be specified Forms or checklists may be provided and required Sometimes included with your application; sometimes sent or uploaded separately • Provide your current full CV. • Provide a good draft of the proposal. • Talk with the writer about your long term plans and goals. • Provide a draft letter giving an overview of the project and your career goals. Include any elements you want to see in the letter. 74

You may also need a letter from your Chair, the Dean, or the President • The Chair may know you § Provide draft letter § Provide all information given to others writing letters § Get mentor to help • The Dean and the President of Yale probably don’t know you very well § Don’t panic § The Department, ORA and Development can arrange these and help with writing § Will need information described above § May call you for additional information § Will ask your Mentor or Chair for draft letter 75

More on letters • Don’t be shy about asking for letters § It’s part of the senior faculty’s job to mentor you and do these things § Make their job as easy as possible § Approach them early - give them enough time • Multiple requests are not a problem § Second and subsequent letters are easy § Computers are our friends • Be sure to let your writers know when you get an award 76

Readers • Begin asking people to read the grant at an early draft stage (~2 months before submission) • Use their input and feedback as you develop the project • Do this early enough that you can add or delete experiments, aims, and collaborators • Projects evolve while they are being written. Allow time to re-write when this happens 77

Who should read the final drafts? • All collaborators must have an opportunity to read the proposal (ethical issue!) • Anyone who is writing a letter for you should be given a good draft • Outside readers (at least 3!) § An expert in the field § A person in a closely related field § An intelligent non-expert Good proofreader; good English skills This reader will provide a critical perspective if there are non-scientists on the review panel 78

A few words on readers • You want people who are honest and critical • You want both scientific comments and editorial comments • Pick people who will take the time to read thoroughly and thoughtfully • Yes, it is an imposition to ask a senior colleague to read your grant § Ask anyhow § It’s part of their job § Give them enough time • With your peers: trade favors 79

The final proofreading • Use spell check program • Use grammar check program • Don’t trust the programs! Proofread! § “principle investigator” § “hear at Yael, wee all ways proof reed. ” • Have multiple people proofread • Check figures, tables, data, legends • If your English skills are not strong, get someone with strong English writing skills to edit and proofread for you • If needed: contact the library for help finding professional editing services 80

Assembling the application • Did you include everything the grantmaker said to include? • Follow the instructions to the letter § Where/how to number pages? § What order? § How to handle appendices? • If electronic § One file or several? § What kind of file (Adobe? Word? A web-based form? ) • If paper: § How many copies? § Staple copies or not? • Identify proprietary or confidential information? • You don’t want the application refused because you sent it in the wrong format !! 81

Sending the application • • • Paper or electronic? Where? When? How? With cover letter? § It depends on your sponsor § May be required, with specific information included § You may be able to request consideration by a specific institute or review by a specific review committee § You may be able to request that specific people not review the application • Watch for special instructions 82

Electronic submission: still a major problem • Overload near grant deadlines slows systems, prevents uploading, and causes systems to crash • Grants can just disappear into cyberspace • Check uploaded grant: § Open every file § Make sure it’s there, legible, complete, & the right length • For grants. gov submissions – watch for error messages that identify problems requiring correction or resubmission before the submission deadline • Contact GCA for help • Keep following the submission until you have a confirmation of successful receipt 83

Warning : For NIH grants • The two day correction window is gone as of January 25, 2011 • Grants that are not accepted by the NIH Website by the submission deadline (date and time!) will not be considered. • Minor errors can prevent acceptance (e. g. extra spaces, changes to formatting/length during uploading to the website, a section 1 line over the page limit, a minor typo at a critical place). 84

Warnings: Other Sponsors • Most other agencies already have no correction window – if the grant is not completely and correctly uploaded at the exact time of the deadline, it is indeed dead • NSF now requires data management plans • NSF will not review any research grant that proposes cost sharing • NSF now requires a mentoring plan on research grants that support trainees (and RCR training for all trainees) • Every sponsor has its own specific set of requirements and they are changing rapidly 85

It’s submitted. Now what? • You wait and watch for information • May get an acknowledgement and information on assignment for review and contact person (or you may have to check a website) • The review can take months • In some cases you may be asked for additional information - send it ASAP • In some cases you may wish to send new information - contact the grantmaker before sending anything 86

A final word • All sponsors receive many more applications than they can fund § § NIH (2010): received 65, 010 / funded 14, 659 NSF: receives ~40, 000 / funds ~11, 000 Am Cancer Soc: receives ~ 2000 / funds ~260 Brown Cox (2009): received 65 / funded 13 • Each review panel reviews 80 -100 grants per session • Each reviewer gets 5 -20 grants to read • Regardless of sponsor, make your grant the best one your reviewer reads, so he or she fights to get it funded 87

Questions? 88