Writing neutralization equations When acids and bases are

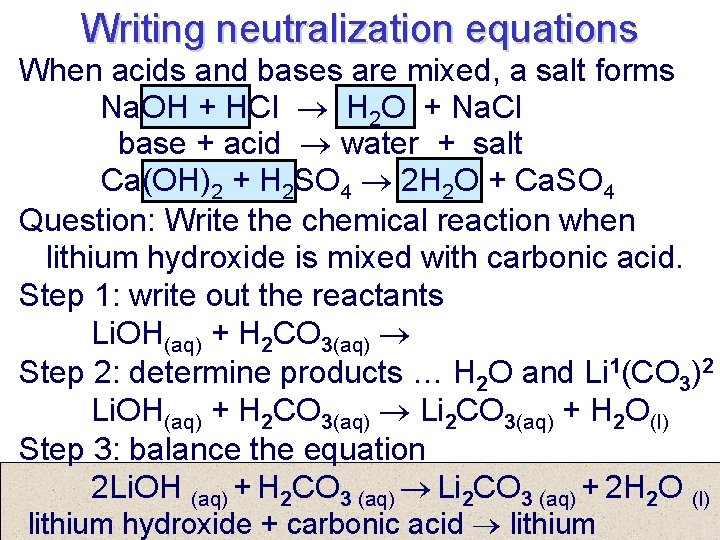
Writing neutralization equations When acids and bases are mixed, a salt forms Na. OH + HCl H 2 O + Na. Cl base + acid water + salt Ca(OH)2 + H 2 SO 4 2 H 2 O + Ca. SO 4 Question: Write the chemical reaction when lithium hydroxide is mixed with carbonic acid. Step 1: write out the reactants Li. OH(aq) + H 2 CO 3(aq) Step 2: determine products … H 2 O and Li 1(CO 3)2 Li. OH(aq) + H 2 CO 3(aq) Li 2 CO 3(aq) + H 2 O(l) Step 3: balance the equation 2 Li. OH (aq) + H 2 CO 3 (aq) Li 2 CO 3 (aq) + 2 H 2 O (l) lithium hydroxide + carbonic acid lithium

Assignment Write balanced chemical equations for these neutralization reactions. Under each compound give the correct (IUPAC) name. • • • iron(II) hydroxide + phosphoric acid Ba(OH)2(aq) + HCl(aq) calcium hydroxide + nitric acid Al(OH)3(aq) + H 2 SO 4(aq) ammonium hydroxide + hydrosulfuric acid KOH(aq) + HCl. O 2(aq)

a) 3 Fe(OH)2(aq) + 2 H 3 PO 4(aq) Fe 3(PO 4)2(aq) + 6 H 2 O(l) iron(II) hydroxide + phosphoric acid iron (II) phosphate b) Ba(OH)2(aq) + 2 HCl(aq) Ba. Cl 2 (aq) + 2 H 2 O(l) barium hydroxide + hydrochloric acid barium chloride c) Ca(OH)2(aq) + 2 HNO 3(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) calcium hydroxide + nitric acid calcium nitrate d) 2 Al(OH)3(aq) + 3 H 2 SO 4(aq) Al 2(SO 4)3(aq) + 6 H 2 O(l) aluminum hydroxide + sulfuric acid aluminum sulfate
- Slides: 4