Writing Lewis Structures Lewis structures Step 1 Total
- Slides: 22

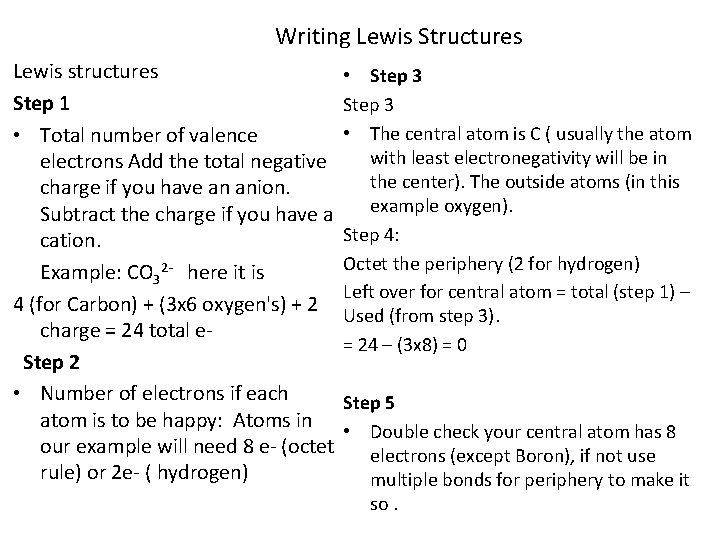
Writing Lewis Structures Lewis structures Step 1 • Total number of valence electrons Add the total negative charge if you have an anion. Subtract the charge if you have a cation. Example: CO 32 - here it is 4 (for Carbon) + (3 x 6 oxygen's) + 2 charge = 24 total e. Step 2 • Number of electrons if each atom is to be happy: Atoms in our example will need 8 e- (octet rule) or 2 e- ( hydrogen) • Step 3 • The central atom is C ( usually the atom with least electronegativity will be in the center). The outside atoms (in this example oxygen). Step 4: Octet the periphery (2 for hydrogen) Left over for central atom = total (step 1) – Used (from step 3). = 24 – (3 x 8) = 0 Step 5 • Double check your central atom has 8 electrons (except Boron), if not use multiple bonds for periphery to make it so.

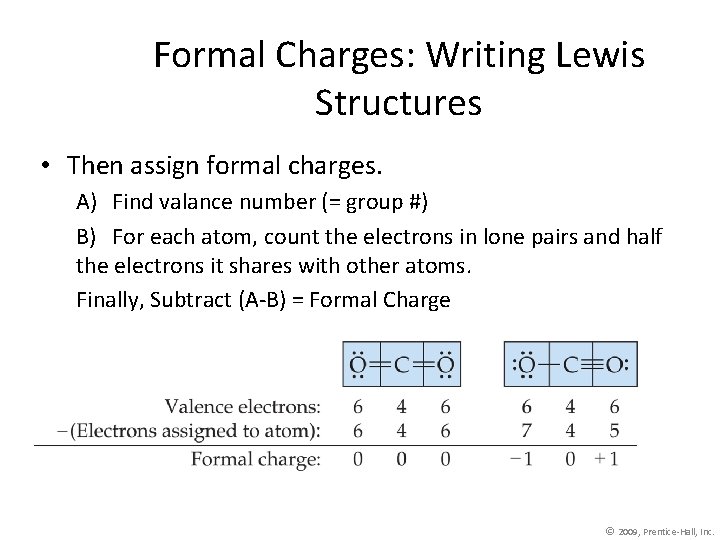
Formal Charges: Writing Lewis Structures • Then assign formal charges. A) Find valance number (= group #) B) For each atom, count the electrons in lone pairs and half the electrons it shares with other atoms. Finally, Subtract (A-B) = Formal Charge © 2009, Prentice-Hall, Inc.

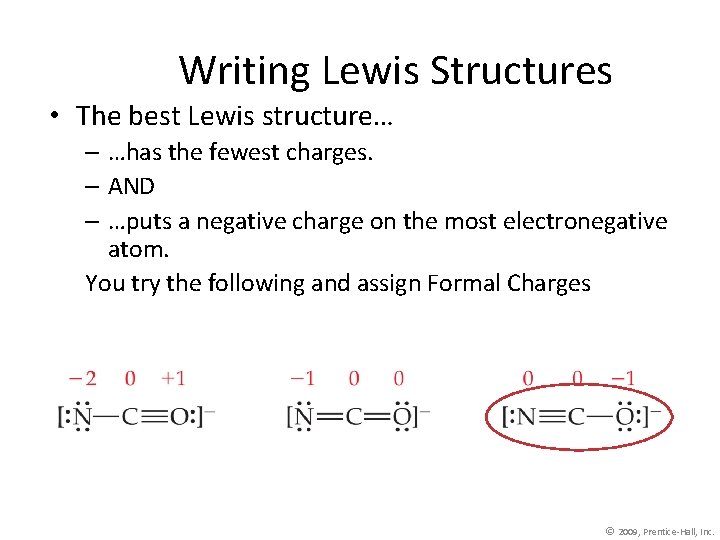
Writing Lewis Structures • The best Lewis structure… – …has the fewest charges. – AND – …puts a negative charge on the most electronegative atom. You try the following and assign Formal Charges © 2009, Prentice-Hall, Inc.

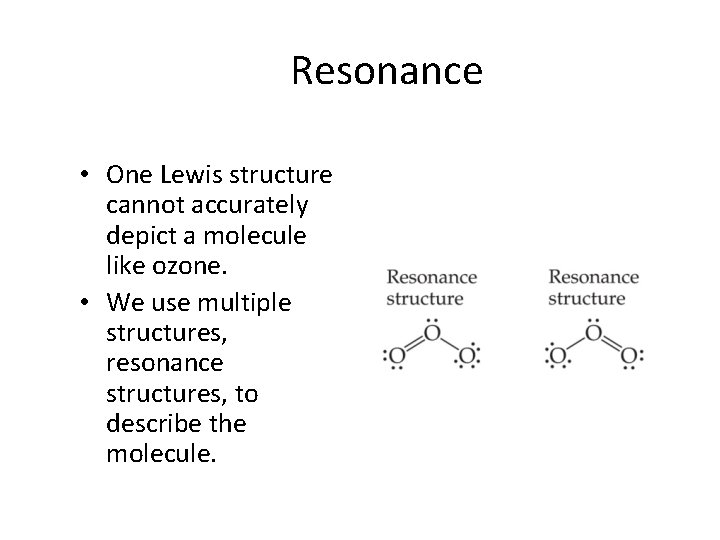
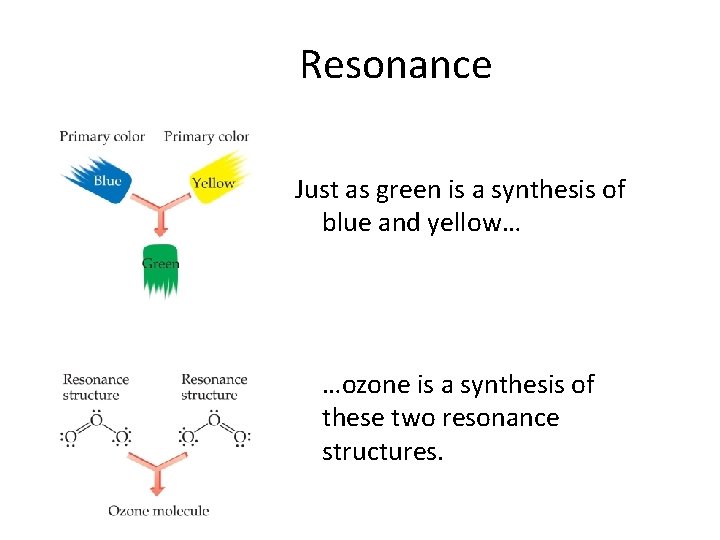
Resonance • One Lewis structure cannot accurately depict a molecule like ozone. • We use multiple structures, resonance structures, to describe the molecule.

Resonance Just as green is a synthesis of blue and yellow… …ozone is a synthesis of these two resonance structures.

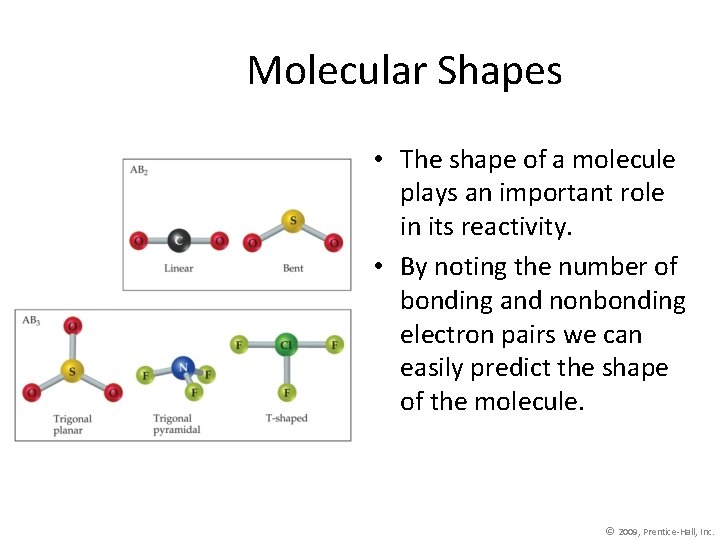
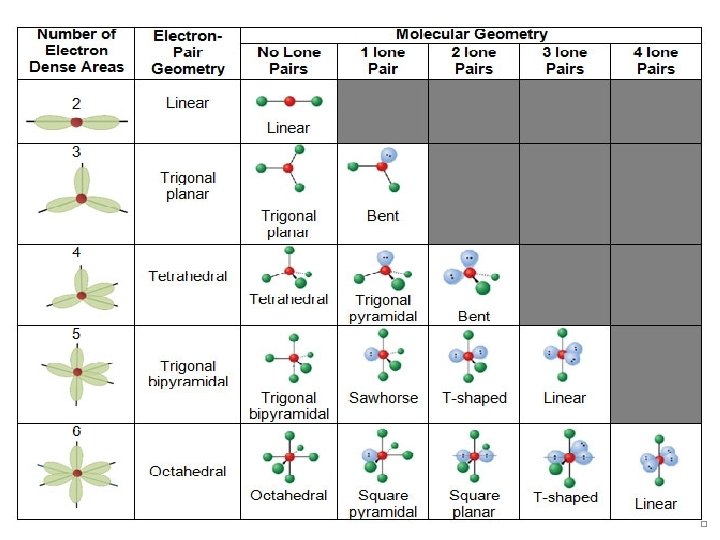
Molecular Shapes • The shape of a molecule plays an important role in its reactivity. • By noting the number of bonding and nonbonding electron pairs we can easily predict the shape of the molecule. © 2009, Prentice-Hall, Inc.

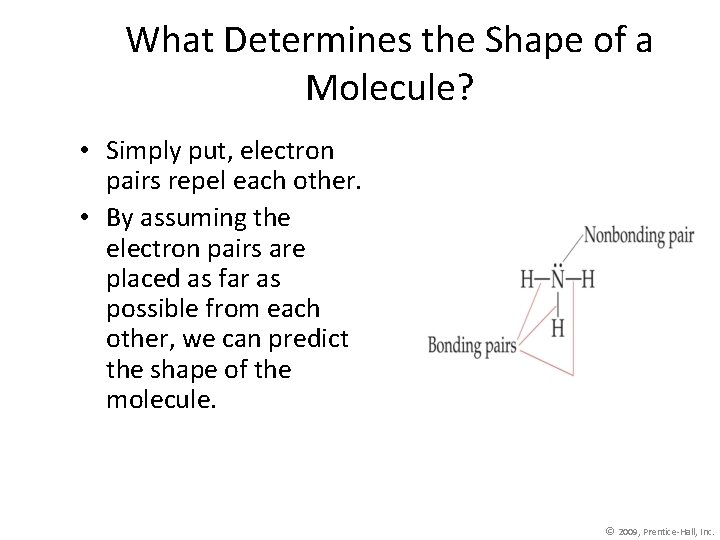
What Determines the Shape of a Molecule? • Simply put, electron pairs repel each other. • By assuming the electron pairs are placed as far as possible from each other, we can predict the shape of the molecule. © 2009, Prentice-Hall, Inc.

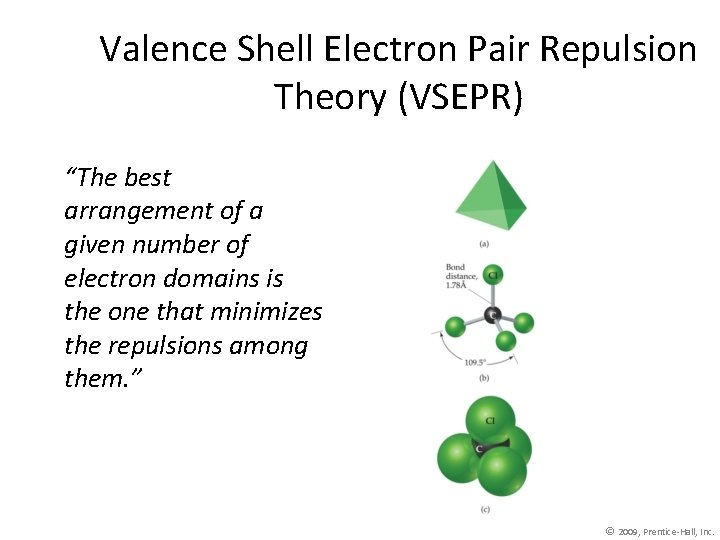
Valence Shell Electron Pair Repulsion Theory (VSEPR) “The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them. ” © 2009, Prentice-Hall, Inc.

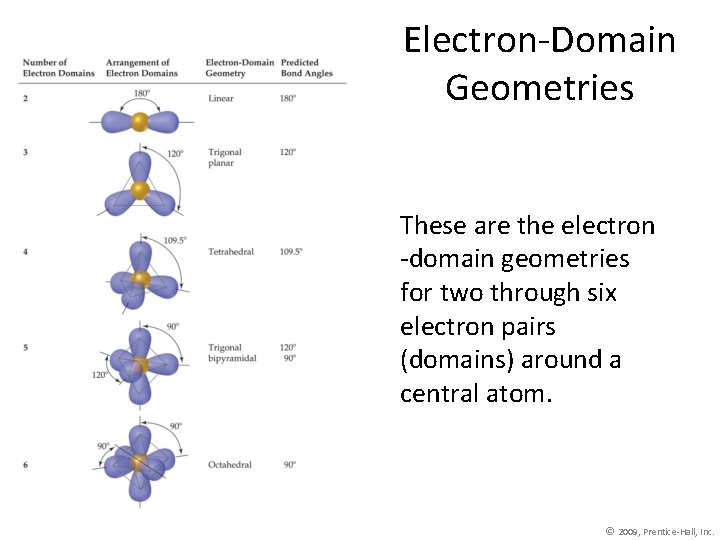
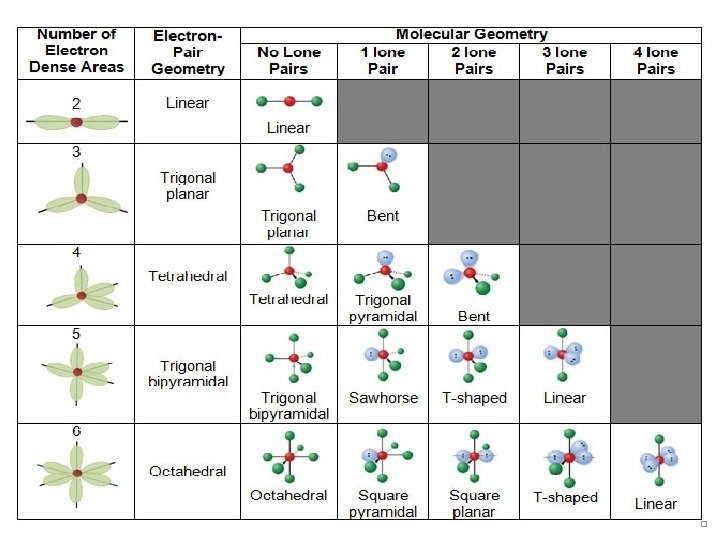
Electron-Domain Geometries These are the electron -domain geometries for two through six electron pairs (domains) around a central atom. © 2009, Prentice-Hall, Inc.




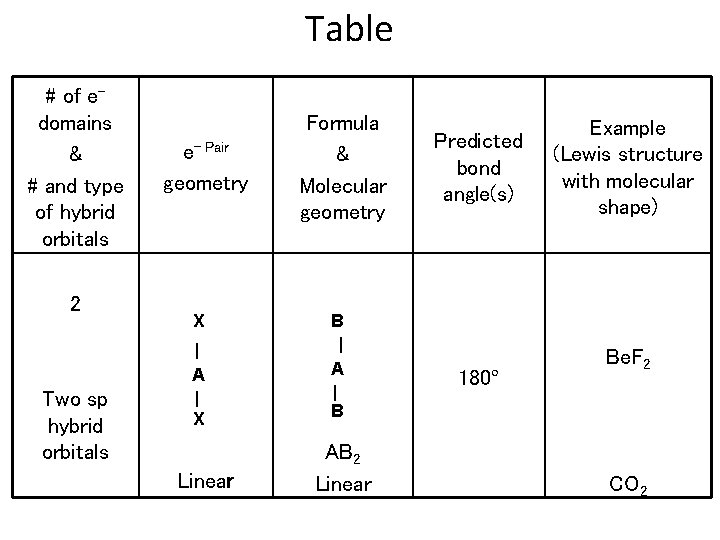
Table # of edomains & # and type of hybrid orbitals 2 Two sp hybrid orbitals e- Pair geometry X | A | X Linear Formula & Molecular geometry B | A | B AB 2 Linear Predicted bond angle(s) 180º Example (Lewis structure with molecular shape) Be. F 2 CO 2

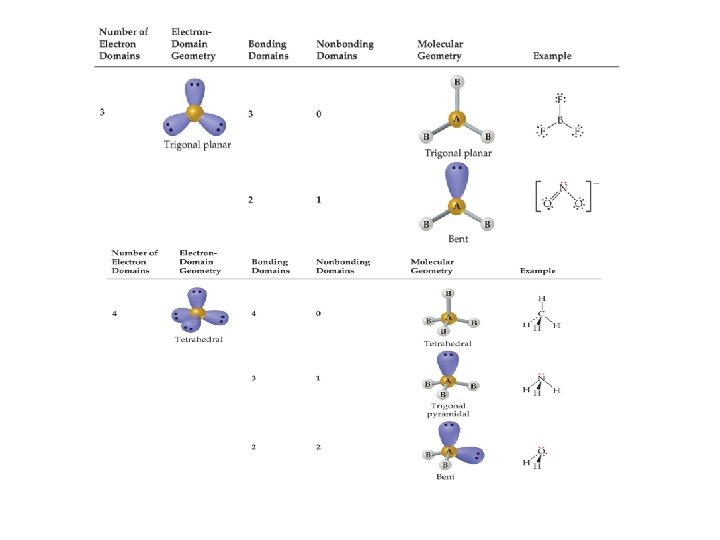
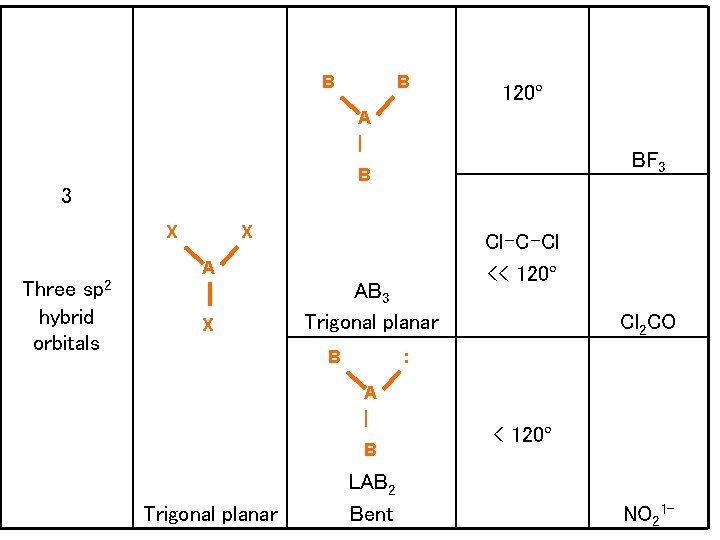
B B 120º A | B 3 X Three sp 2 hybrid orbitals BF 3 X AB 3 Trigonal planar B Cl 2 CO : A | B Trigonal planar Cl-C-Cl << 120º LAB 2 Bent < 120º NO 21 -

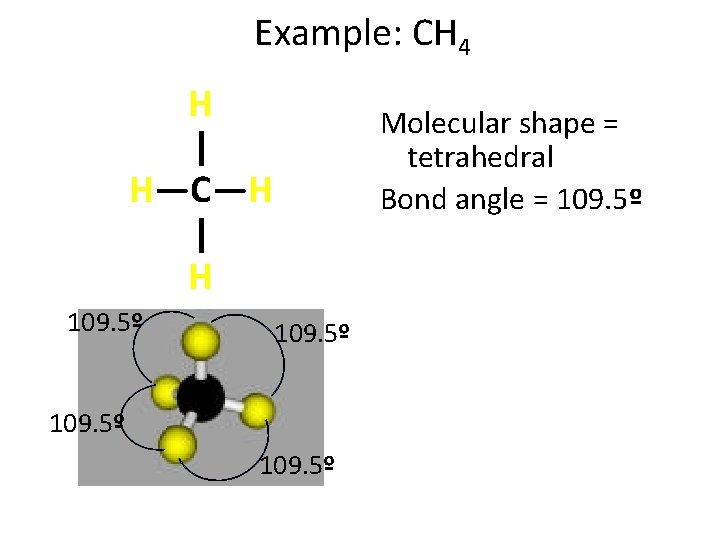
Example: CH 4 H | H—C—H | H 109. 5º Molecular shape = tetrahedral Bond angle = 109. 5º

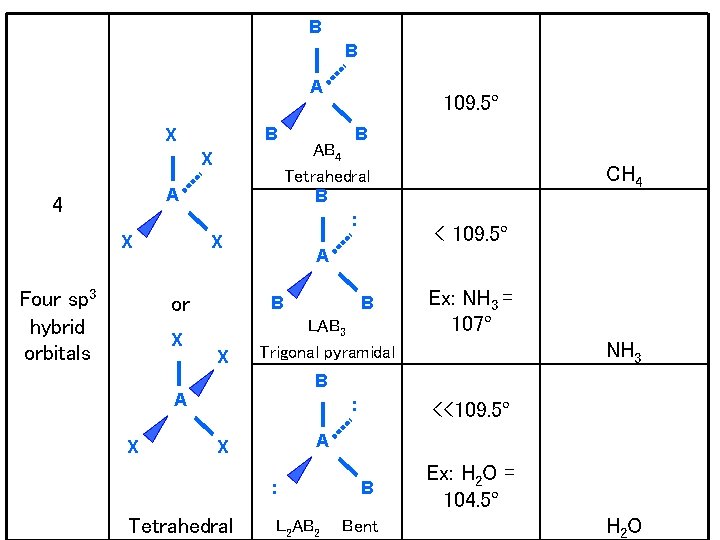
B B A B X X A 4 X Four sp 3 hybrid orbitals X B X CH 4 < 109. 5º A B LAB 3 Trigonal pyramidal Ex: NH 3 = 107º NH 3 B A X B AB 4 Tetrahedral B : X or 109. 5º : A X : Tetrahedral <<109. 5º L 2 AB 2 B Bent Ex: H 2 O = 104. 5º H 2 O

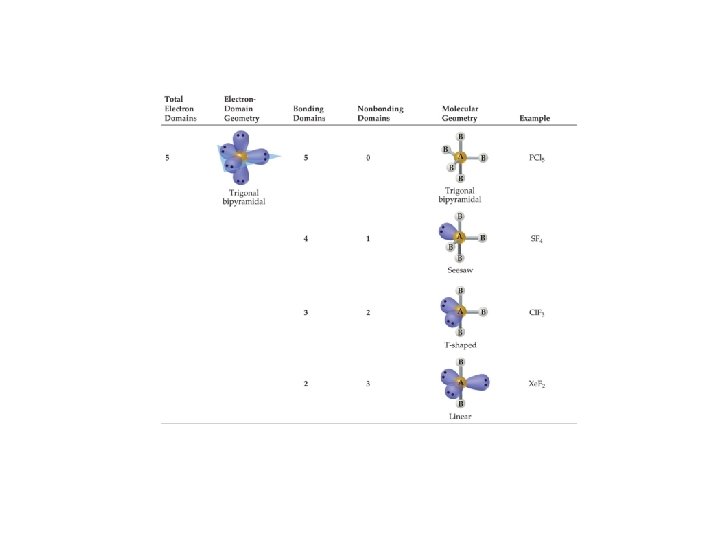
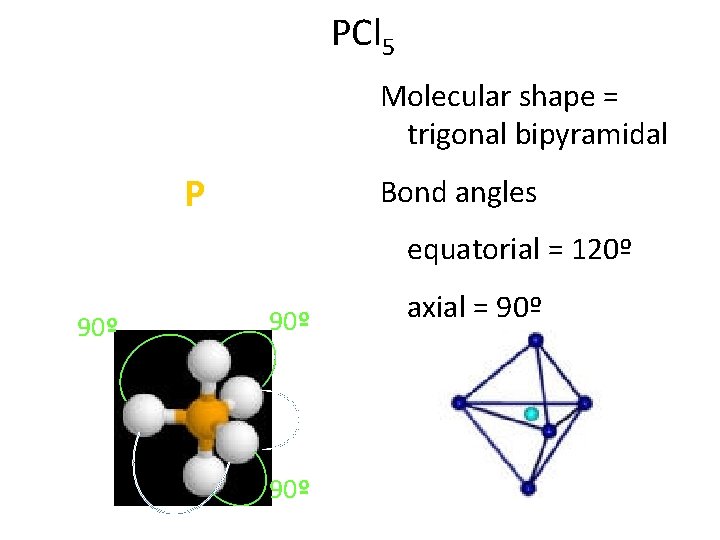
PCl 5 : : Molecular shape = trigonal bipyramidal : : : Cl: / : Cl—P—Cl: | : Cl: : 90º equatorial = 120º 90º 120º Bond angles 90º axial = 90º

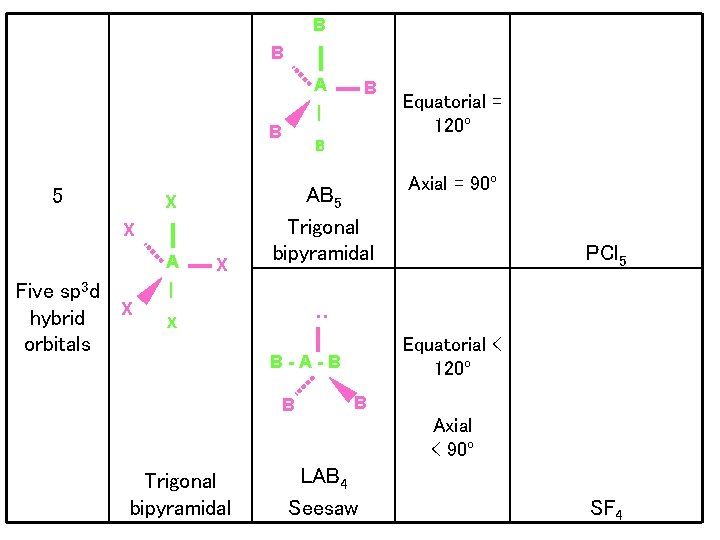
B B A | B X X A Five sp 3 d X hybrid orbitals X B AB 5 Trigonal bipyramidal Equatorial = 120º Axial = 90º PCl 5 | X : 5 B Equatorial < 120º B-A-B B B Axial < 90º Trigonal bipyramidal LAB 4 Seesaw SF 4

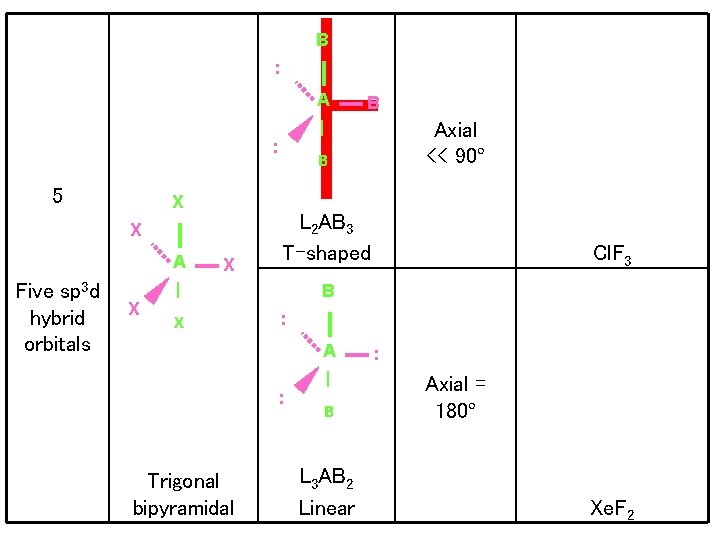
B : A | : 5 X X A Five sp 3 d hybrid orbitals X X L 2 AB 3 T-shaped Cl. F 3 B : A : Trigonal bipyramidal Axial << 90º B | X B | B L 3 AB 2 Linear : Axial = 180º Xe. F 2

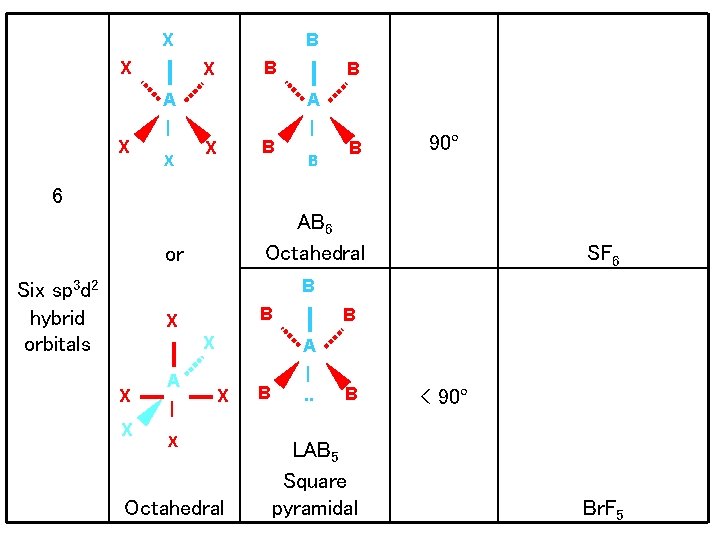
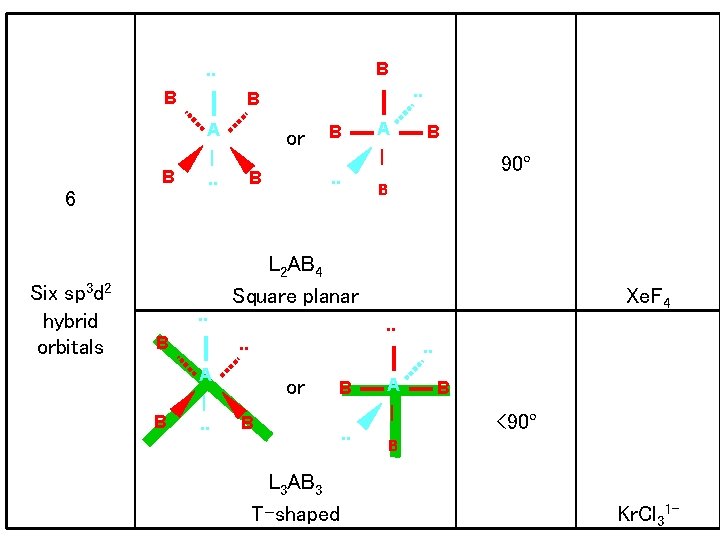
X X X B B X B A A | | X B B 90º 6 AB 6 Octahedral or SF 6 B Six sp 3 d 2 hybrid orbitals B X X A | B A X X Octahedral B |. . B LAB 5 Square pyramidal < 90º Br. F 5

B . . B A or |. . B 6 Six sp 3 d 2 hybrid orbitals . . B B |. . 90º B Xe. F 4. . |. . A L 2 AB 4 Square planar. . A B . . B or B B L 3 AB 3 T-shaped A | . . B <90º B Kr. Cl 31 -
