Writing Ionic Formulas Rules for Writing Formulas O




- Slides: 8

Writing Ionic Formulas

Rules for Writing Formulas O Ionic compounds are formed when metals form O O bonds with nonmetals. Electrons are lost by the metal and gained by the nonmetals. Cations bond with anions. Ionic compounds are neutral in total charge so your positive and negative charges must balance. Positive and negative charges of the ions must be compared to write a correct formula. They should not be in the formula. Transition metal charges are indicated by roman numerals in the names.

Rules for Writing Formulas O Write the ionic symbols of the elements (symbol and it’s charge) O Compare the charges of the cation and anion. O If they cancel, get rid of the charges and leave the symbols for the formula. O If they do not cancel, use the criss cross method to determine the subscripts needed.

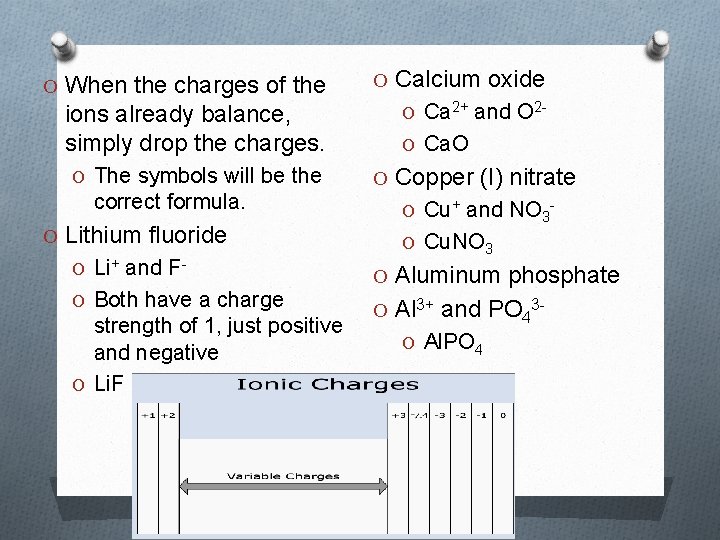
O When the charges of the O Calcium oxide ions already balance, simply drop the charges. O Ca 2+ and O 2 - O The symbols will be the O Copper (I) nitrate correct formula. O Lithium fluoride O Li+ and FO Both have a charge strength of 1, just positive and negative O Li. F O Ca. O O Cu+ and NO 3 O Cu. NO 3 O Aluminum phosphate O Al 3+ and PO 43 O Al. PO 4

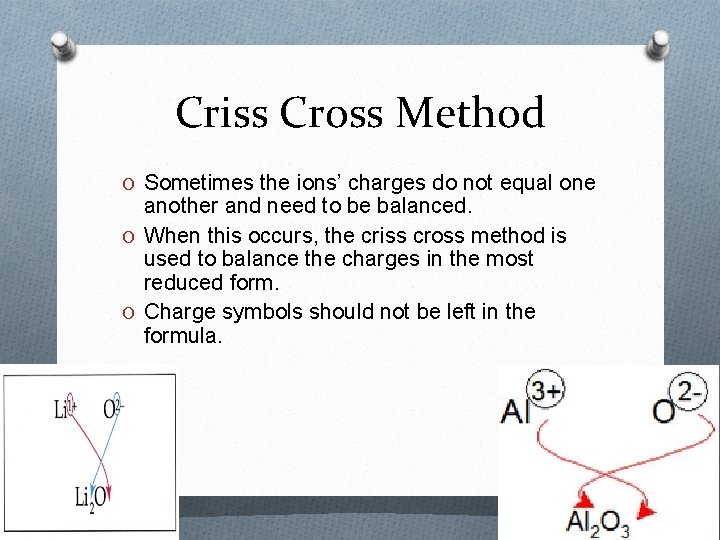
Criss Cross Method O Sometimes the ions’ charges do not equal one another and need to be balanced. O When this occurs, the criss cross method is used to balance the charges in the most reduced form. O Charge symbols should not be left in the formula.

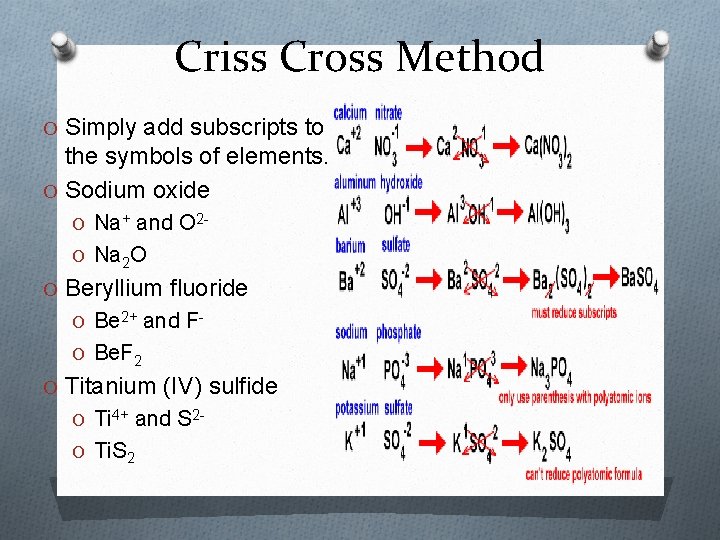
Criss Cross Method O Simply add subscripts to the symbols of elements. O Sodium oxide O Na+ and O 2 O Na 2 O O Beryllium fluoride O Be 2+ and FO Be. F 2 O Titanium (IV) sulfide O Ti 4+ and S 2 O Ti. S 2

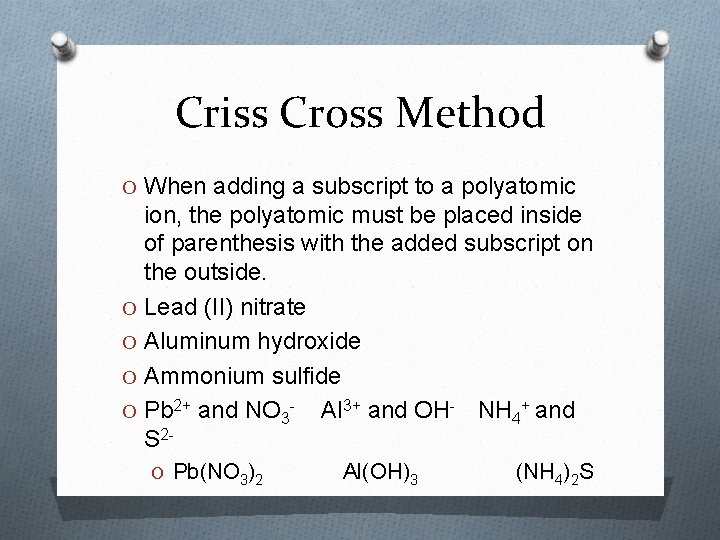
Criss Cross Method O When adding a subscript to a polyatomic ion, the polyatomic must be placed inside of parenthesis with the added subscript on the outside. O Lead (II) nitrate O Aluminum hydroxide O Ammonium sulfide O Pb 2+ and NO 3 - Al 3+ and OH- NH 4+ and S 2 O Pb(NO 3)2 Al(OH)3 (NH 4)2 S

O Lithium fluoride O Ammonium oxide O Magnesium sulfite O Copper (II) phosphite O Calcium nitrate O Lead (IV) sulfate