Writing Formulas and Naming Compounds Binary Compounds Compounds
Writing Formulas and Naming Compounds
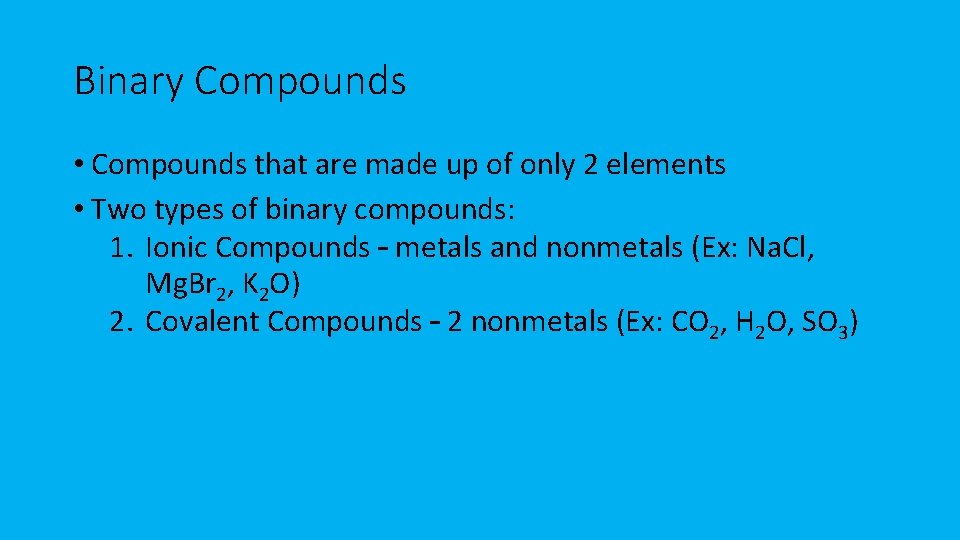
Binary Compounds • Compounds that are made up of only 2 elements • Two types of binary compounds: 1. Ionic Compounds – metals and nonmetals (Ex: Na. Cl, Mg. Br 2, K 2 O) 2. Covalent Compounds – 2 nonmetals (Ex: CO 2, H 2 O, SO 3)

Practice – Ionic or Covalent? 1. 2. 3. 4. NO 2 P 2 O 4 Fe 2 O 3 Ca. F 2 covalent ionic
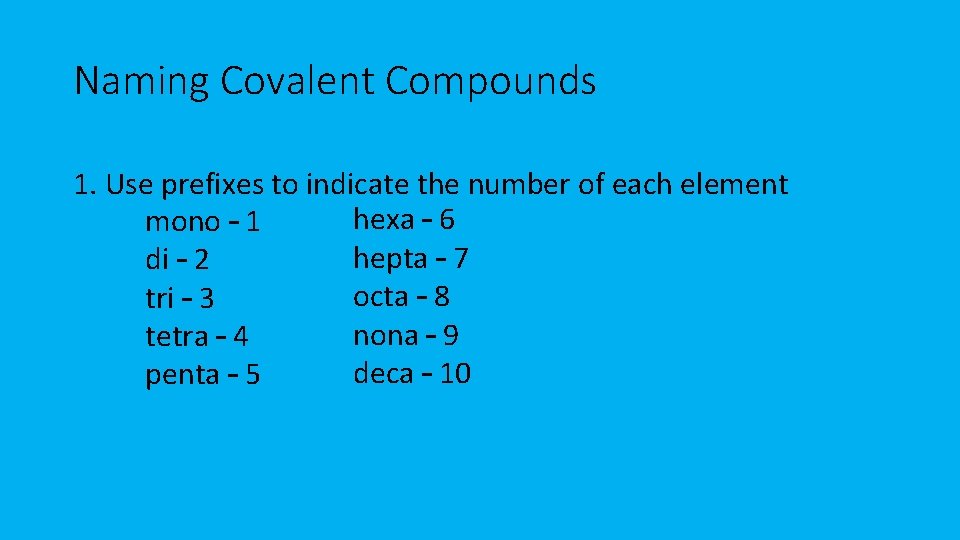
Naming Covalent Compounds 1. Use prefixes to indicate the number of each element hexa – 6 mono – 1 hepta – 7 di – 2 octa – 8 tri – 3 nona – 9 tetra – 4 deca – 10 penta – 5

Naming Covalent Compounds 2. Name the first element. If you have only one atom of the first element, do NOT use the prefix mono-. 3. Name the second element with prefixes. Change the ending to “-ide. ”

Naming Covalent Compounds - Practice 1. CO 2 carbon dioxide 2. SO 3 sulfur trioxide 3. N 2 O 4 dinitrogen tetroxide 4. CCl 4 carbon tetrachloride 5. H 2 O dihydrogen monoxide

Writing Covalent Compounds 1. phosphorus trichloride PCl 3 2. nitrogen dioxide NO 2 3. sulfur hexabromide SBr 6 4. diphosphorus pentoxide P 2 O 5

Naming Ionic Compounds 1. Name the first element (metal) 2. Name the second element (nonmetal) and change the ending to ”-ide” 3. NO PREFIXES!!!

Naming Ionic Compounds - Practice 1. Na. Cl sodium chloride 2. Ca. F 2 calcium fluoride 3. Ag 2 O silver oxide 4. Al. Br 3 aluminum bromide 5. Zn. S zinc sulfide

Writing Ionic Formulas Ionic compounds are composed of a positive ion called a cation and a negative ion called an anion. 1. Write the metal ion first ( + ion: cation) 2. Write the nonmetal last ( - ion: anion) 3. Balance the charges! Charges must add up to zero to form a neutral compound.
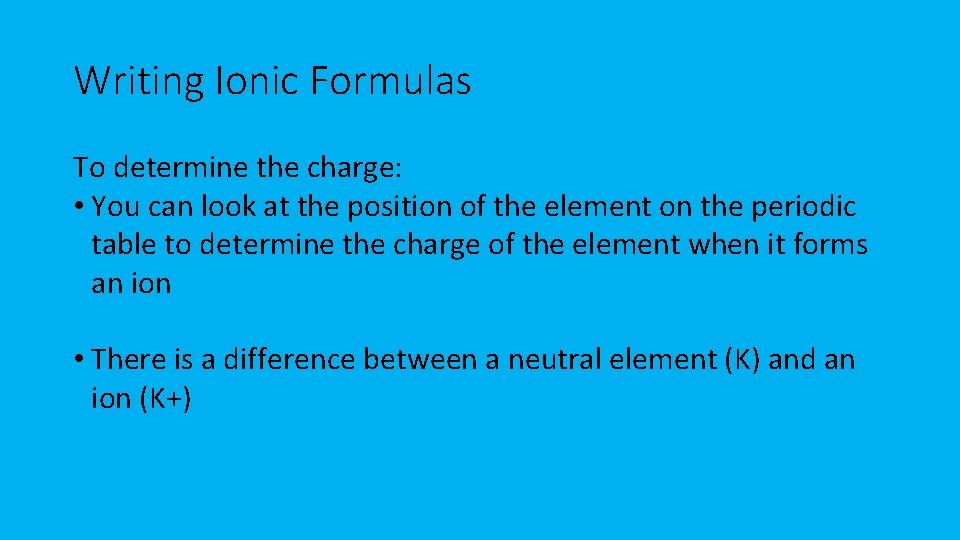
Writing Ionic Formulas To determine the charge: • You can look at the position of the element on the periodic table to determine the charge of the element when it forms an ion • There is a difference between a neutral element (K) and an ion (K+)
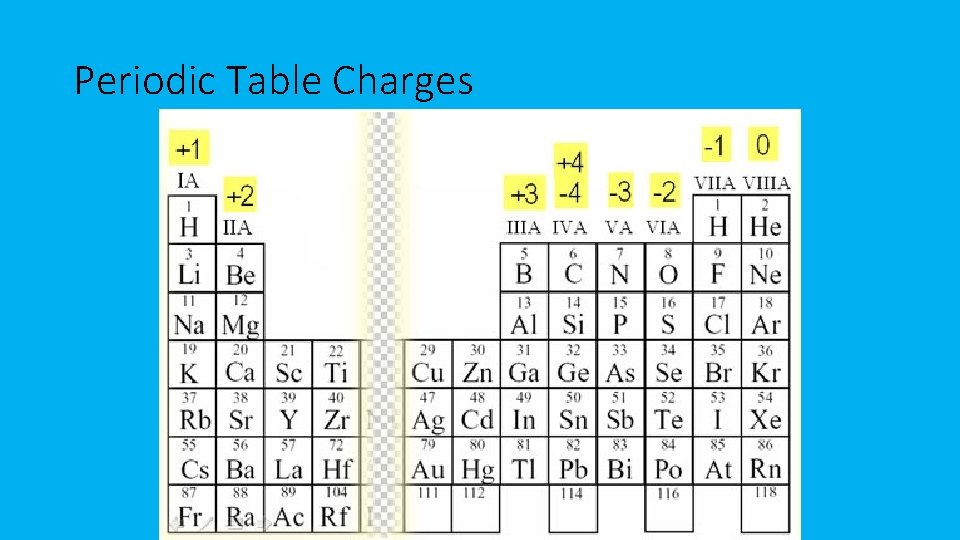
Periodic Table Charges

Writing Ionic Compound Formulas 1. sodium chloride Na+ Cl- Na. Cl 2. calcium chloride Ca+2 Cl. Cl- Ca. Cl 2

Writing Ionic Compound Formulas 3. aluminum chloride Al+3 Cl. Cl. Al. Cl 3

Writing Ionic Compound Formulas 4. potassium bromide K+ Br. KBr 5. potassium oxide K+ O-2 K+ K 2 O

Writing Ionic Compound Formulas 6. aluminum oxide O-2 Al+3 -2 +3 O Al O-2 Al 2 O 3

Ionic Compounds with Roman Numerals • The roman numeral gives the charge of the metal ion. • Ex: Fe(II) Fe+2 Fe(III) Fe+3 • Transition metals need roman numerals in the name. • Pb and Sn need roman numerals because they have charges of +2 and +4. • Ag and Zn do NOT need roman numerals because Ag is always +1 and Zn is always +2.

Ionic Compounds with Roman Numerals – Write the Formula 1. nickel (II) bromide Ni. Br 2 2. lead (IV) oxide Pb. O 2 3. iron (III) sulfide Fe 2 S 3

Ionic Compounds with Roman Numerals – Name the Following 1. Cr. Cl 3 chromium (III) chloride 2. Fe. O iron (II) oxide 3. Sn. Cl 4 tin (IV) chloride 4. Cu 2 O copper (I) oxide

Review: Write the Formula 1. strontium fluoride Sr. F 2 2. chromium (III) oxide Cr 2 O 3 3. sulfur hexabromide SBr 6 4. iron (III) sulfide Fe 2 S 3 5. carbon disulfide CS 2

Review: Name the Following. 1. K 2 Se potassium selenide 2. Ca. I 2 calcium iodide 3. Ni. Cl 2 nickel (II) chloride 4. PCl 3 phosphorus trichloride 5. Ba. Na 2 banana

Naming Compounds with Polyatomic Ions 1. Name the metal 2. Name the polyatomic ion 3. Use roman numeral if needed

Naming Polyatomic Ions – Practice: Name the Compound 1. Mg. CO 3 magnesium carbonate 2. Na 2 SO 4 sodium sulfate 3. Cu(OH)2 copper (II) hydroxide 4. Zn(C 2 H 3 O 2)2 zinc acetate

Naming Polyatomic Ions – Practice: Write the Formula 1. aluminum nitrate Al(NO 3)3 2. ammonium sulfite (NH 4)2 SO 3 3. iron (III) chlorate Fe(Cl. O 3)3 4. calcium phosphate Ca 3(PO 4)2


Review: • Covalent compounds are made up of nonmetals only. • Use prefixes in the names of covalent compounds. • Covalent compounds do not have charges! • Ionic compounds are made up of a metal and a nonmetal. • Balance the charges when you write a formula for ionic compounds. • Never use prefixes in the names of ionic compounds!
- Slides: 26