WRITING CHEMICAL EQUATIONS Reactants starting materials Products ending



- Slides: 18

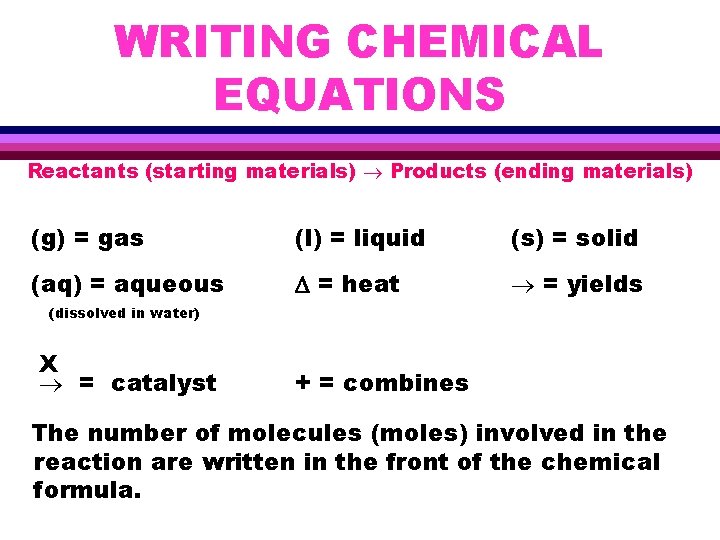
WRITING CHEMICAL EQUATIONS Reactants (starting materials) Products (ending materials) (g) = gas (l) = liquid (s) = solid (aq) = aqueous D = heat = yields (dissolved in water) X = catalyst + = combines The number of molecules (moles) involved in the reaction are written in the front of the chemical formula.

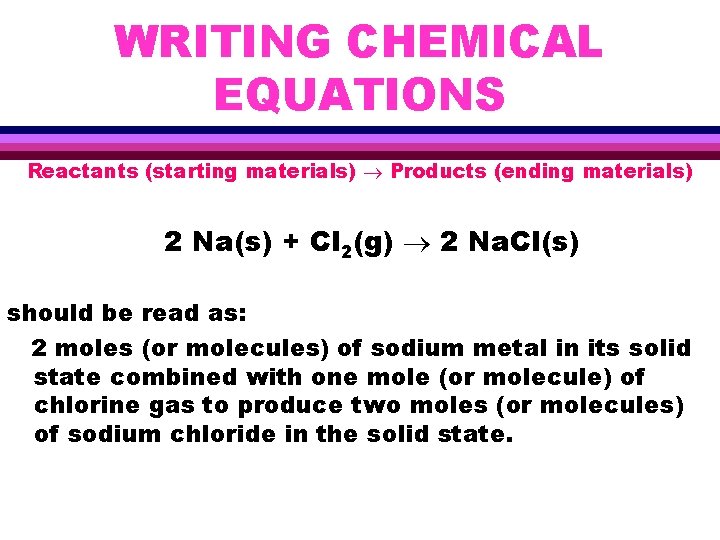
WRITING CHEMICAL EQUATIONS Reactants (starting materials) Products (ending materials) 2 Na(s) + Cl 2(g) 2 Na. Cl(s) should be read as: 2 moles (or molecules) of sodium metal in its solid state combined with one mole (or molecule) of chlorine gas to produce two moles (or molecules) of sodium chloride in the solid state.

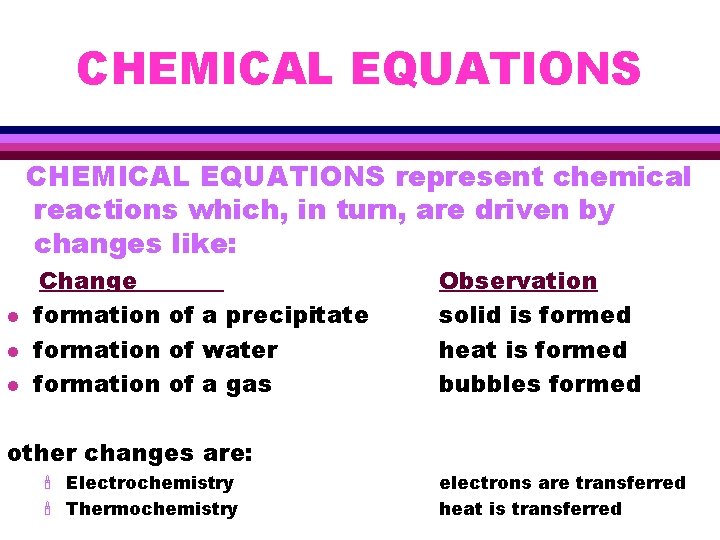
CHEMICAL EQUATIONS represent chemical reactions which, in turn, are driven by changes like: l l l Change formation of a precipitate formation of water formation of a gas Observation solid is formed heat is formed bubbles formed other changes are: ' Electrochemistry ' Thermochemistry electrons are transferred heat is transferred

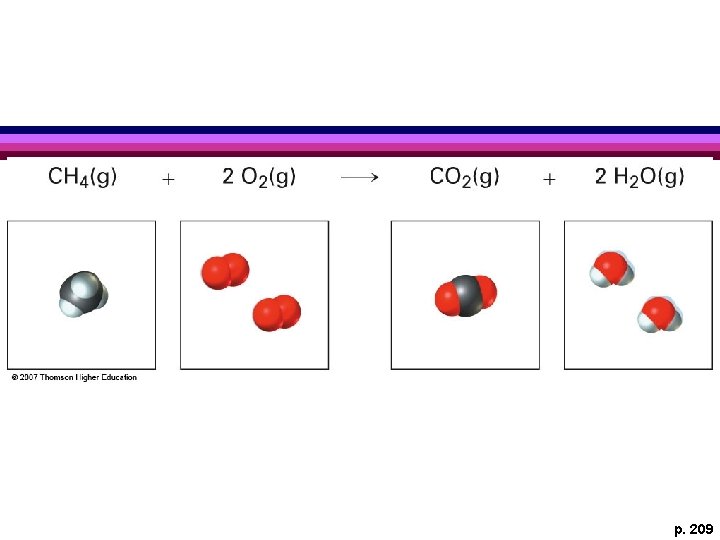
p. 209

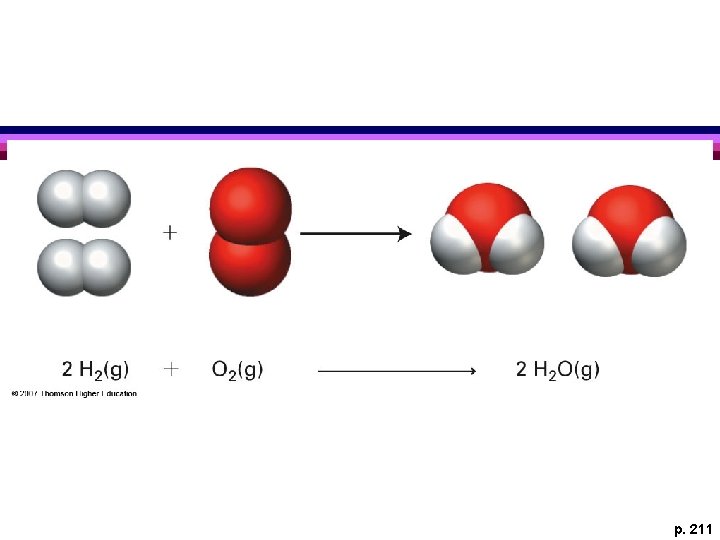
p. 211

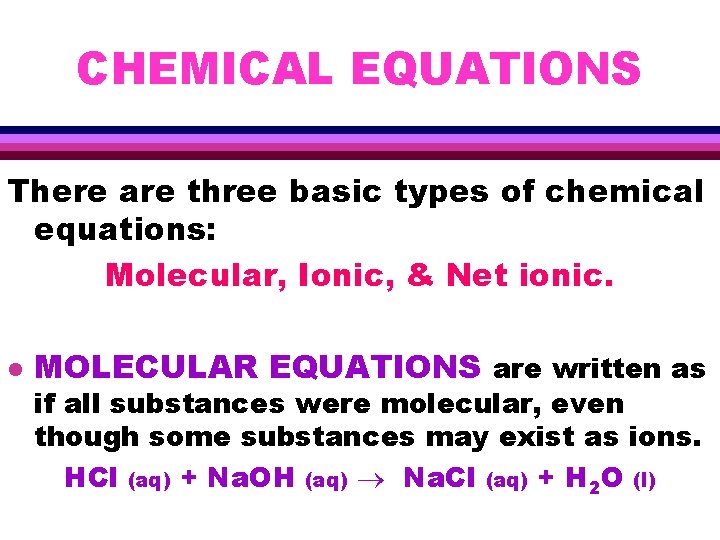
CHEMICAL EQUATIONS There are three basic types of chemical equations: Molecular, Ionic, & Net ionic. l MOLECULAR EQUATIONS are written as if all substances were molecular, even though some substances may exist as ions. HCl (aq) + Na. OH (aq) Na. Cl (aq) + H 2 O (l)

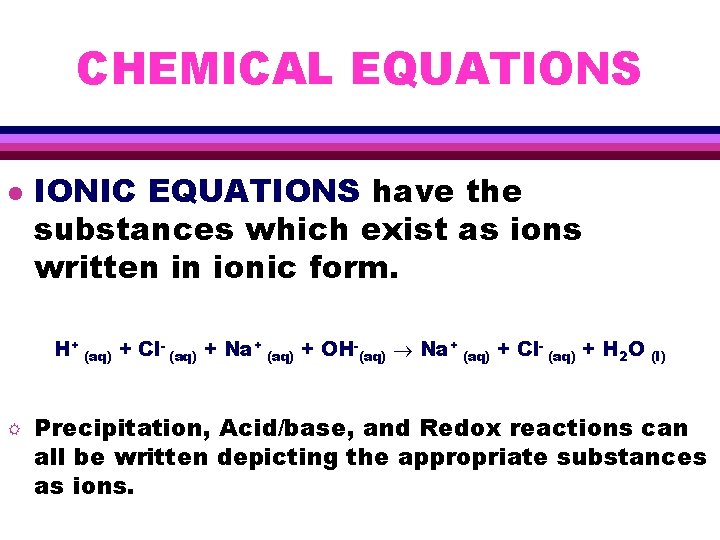
CHEMICAL EQUATIONS l IONIC EQUATIONS have the substances which exist as ions written in ionic form. H+ (aq) + Cl- (aq) + Na+ (aq) + OH-(aq) Na+ (aq) + Cl- (aq) + H 2 O R (l) Precipitation, Acid/base, and Redox reactions can all be written depicting the appropriate substances as ions.

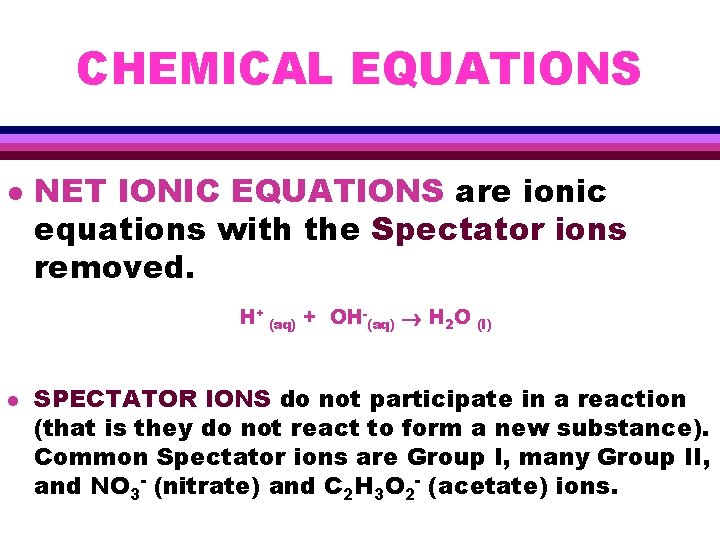
CHEMICAL EQUATIONS l NET IONIC EQUATIONS are ionic equations with the Spectator ions removed. H+ (aq) + OH-(aq) H 2 O (l) l SPECTATOR IONS do not participate in a reaction (that is they do not react to form a new substance). Common Spectator ions are Group I, many Group II, and NO 3 - (nitrate) and C 2 H 3 O 2 - (acetate) ions.

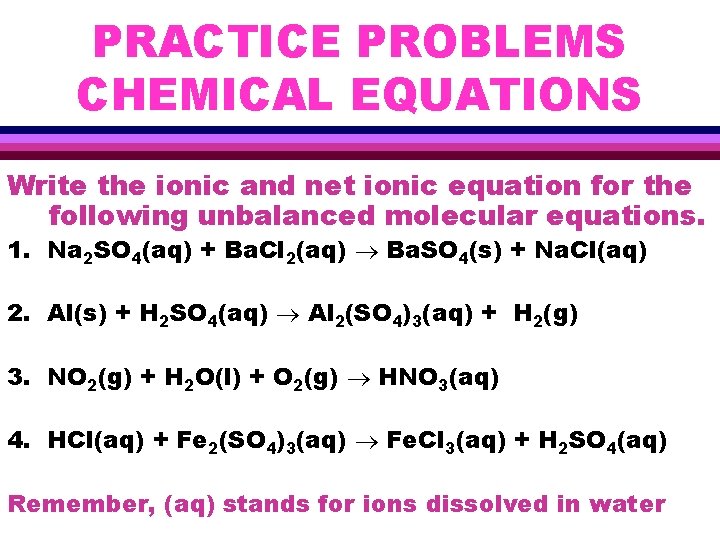
PRACTICE PROBLEMS CHEMICAL EQUATIONS Write the ionic and net ionic equation for the following unbalanced molecular equations. 1. Na 2 SO 4(aq) + Ba. Cl 2(aq) Ba. SO 4(s) + Na. Cl(aq) 2. Al(s) + H 2 SO 4(aq) Al 2(SO 4)3(aq) + H 2(g) 3. NO 2(g) + H 2 O(l) + O 2(g) HNO 3(aq) 4. HCl(aq) + Fe 2(SO 4)3(aq) Fe. Cl 3(aq) + H 2 SO 4(aq) Remember, (aq) stands for ions dissolved in water

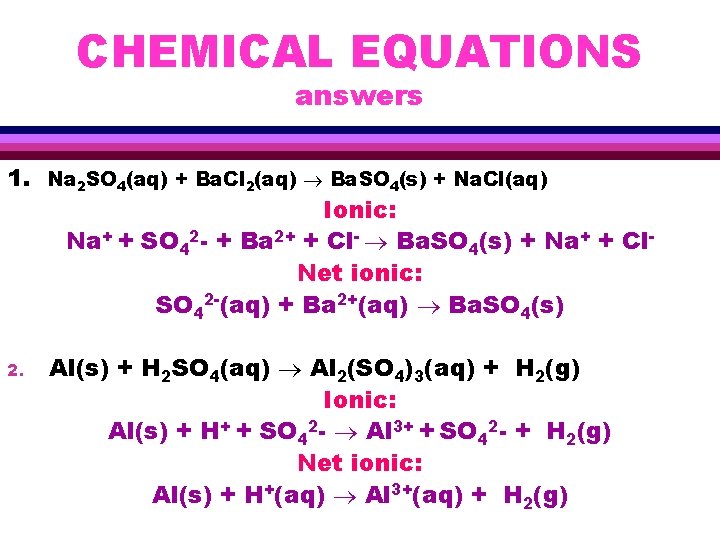
CHEMICAL EQUATIONS answers 1. Na 2 SO 4(aq) + Ba. Cl 2(aq) Ba. SO 4(s) + Na. Cl(aq) Ionic: Na+ + SO 42 - + Ba 2+ + Cl- Ba. SO 4(s) + Na+ + Cl. Net ionic: SO 42 -(aq) + Ba 2+(aq) Ba. SO 4(s) 2. Al(s) + H 2 SO 4(aq) Al 2(SO 4)3(aq) + H 2(g) Ionic: Al(s) + H+ + SO 42 - Al 3+ + SO 42 - + H 2(g) Net ionic: Al(s) + H+(aq) Al 3+(aq) + H 2(g)

CHEMICAL EQUATIONS answers 3. NO 2(g) + H 2 O(l) + O 2(g) HNO 3(aq) NO 2(g) + H 2 O(l) + O 2(g) H+(aq) + NO 3 -(aq) the ionic and net ionic equations are the same! 4. HCl(aq) + Fe 2(SO 4)3(aq) Fe. Cl 3(aq) + H 2 SO 4(aq) H+ + Cl- + Fe 3+ + SO 42 - Fe 3+ + Cl- + H+ + SO 42 - N. R. No reaction occurs! All species are spectator ions.

CHEMICAL EQUATIONS In a chemical reaction, atoms can be neither created nor destroyed therefore the total number of atoms on the reactant side must equal the total number of atoms on the product side of the equation. This is known as BALANCING AN EQUATION. Once the reactants & products have been identified, remember the relative ratio of one atom to another in a formula can not be altered. H 2 O is the formula for water and it is safe to drink but if it is changed to H 2 O 2 (hydrogen peroxide) it is no longer safe for drinking. It is a completely different substance! Equations must be balanced by changing the number in front of the formula and not by changing the chemical formula.

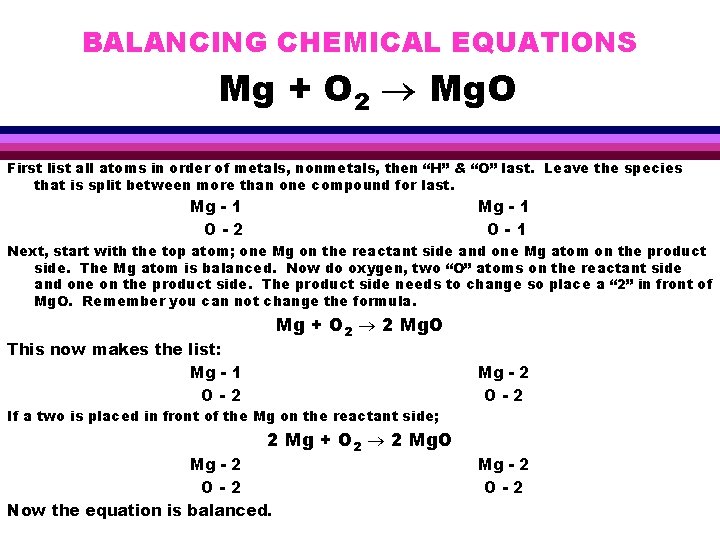
BALANCING CHEMICAL EQUATIONS Mg + O 2 Mg. O First list all atoms in order of metals, nonmetals, then “H” & “O” last. Leave the species that is split between more than one compound for last. Mg - 1 O-2 Mg - 1 O-1 Next, start with the top atom; one Mg on the reactant side and one Mg atom on the product side. The Mg atom is balanced. Now do oxygen, two “O” atoms on the reactant side and one on the product side. The product side needs to change so place a “ 2” in front of Mg. O. Remember you can not change the formula. Mg + O 2 2 Mg. O This now makes the list: Mg - 1 O-2 Mg - 2 O-2 If a two is placed in front of the Mg on the reactant side; 2 Mg + O 2 2 Mg. O Mg - 2 O-2 Now the equation is balanced. Mg - 2 O-2

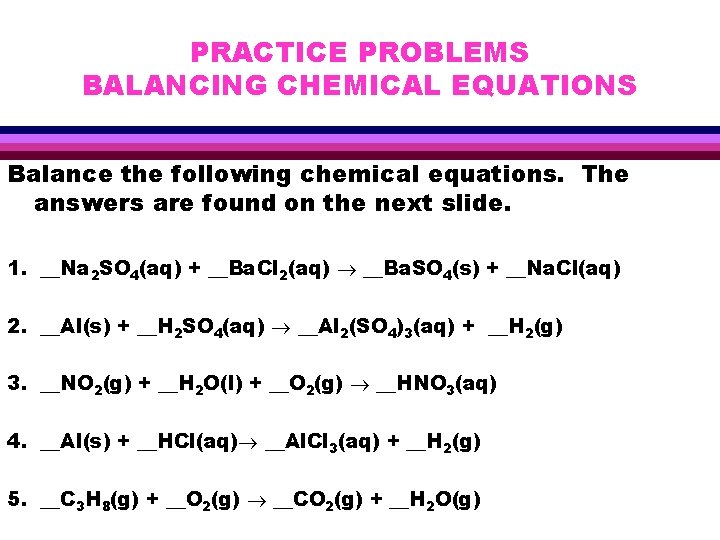
PRACTICE PROBLEMS BALANCING CHEMICAL EQUATIONS Balance the following chemical equations. The answers are found on the next slide. 1. __Na 2 SO 4(aq) + __Ba. Cl 2(aq) __Ba. SO 4(s) + __Na. Cl(aq) 2. __Al(s) + __H 2 SO 4(aq) __Al 2(SO 4)3(aq) + __H 2(g) 3. __NO 2(g) + __H 2 O(l) + __O 2(g) __HNO 3(aq) 4. __Al(s) + __HCl(aq) __Al. Cl 3(aq) + __H 2(g) 5. __C 3 H 8(g) + __O 2(g) __CO 2(g) + __H 2 O(g)

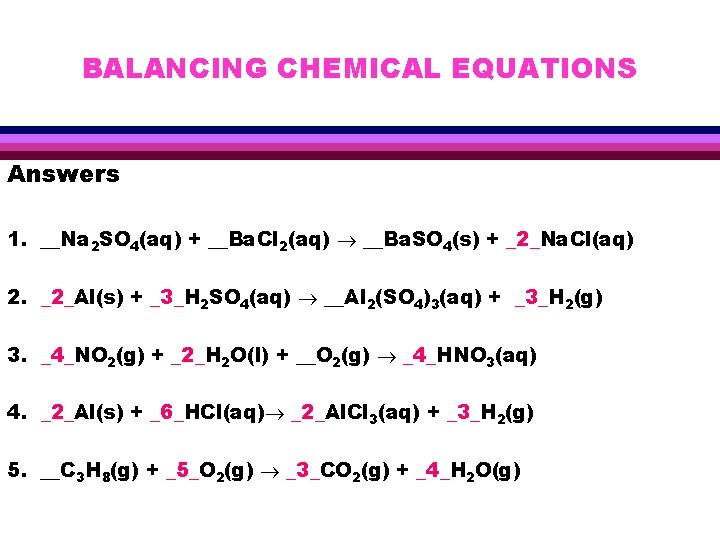
BALANCING CHEMICAL EQUATIONS Answers 1. __Na 2 SO 4(aq) + __Ba. Cl 2(aq) __Ba. SO 4(s) + _2_Na. Cl(aq) 2. _2_Al(s) + _3_H 2 SO 4(aq) __Al 2(SO 4)3(aq) + _3_H 2(g) 3. _4_NO 2(g) + _2_H 2 O(l) + __O 2(g) _4_HNO 3(aq) 4. _2_Al(s) + _6_HCl(aq) _2_Al. Cl 3(aq) + _3_H 2(g) 5. __C 3 H 8(g) + _5_O 2(g) _3_CO 2(g) + _4_H 2 O(g)

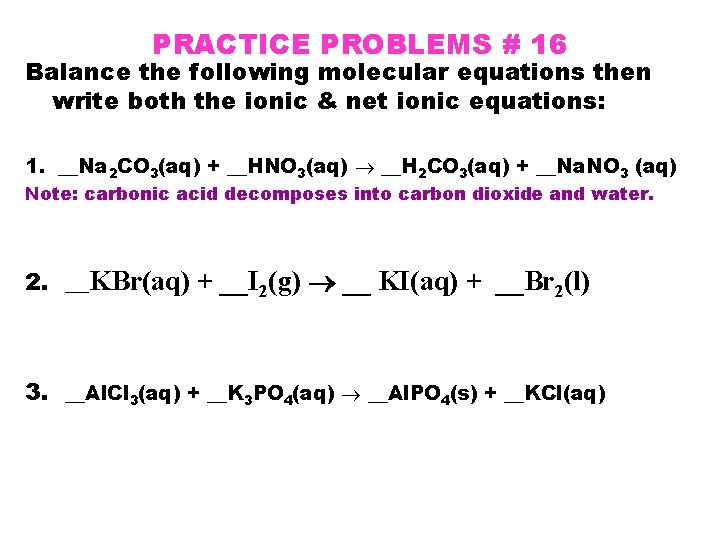
PRACTICE PROBLEMS # 16 Balance the following molecular equations then write both the ionic & net ionic equations: 1. __Na 2 CO 3(aq) + __HNO 3(aq) __H 2 CO 3(aq) + __Na. NO 3 (aq) Note: carbonic acid decomposes into carbon dioxide and water. 2. __KBr(aq) + __I 2(g) __ KI(aq) + __Br 2(l) 3. __Al. Cl 3(aq) + __K 3 PO 4(aq) __Al. PO 4(s) + __KCl(aq)

Answers to PRACTICE PROBLEMS # 16 1. Na 2 CO 3(aq) + 2 HNO 3(aq) H 2 CO 3(aq) + 2 Na. NO 3 (aq) Note: carbonic acid decomposes into carbon dioxide and water. Ionic: Na+ + CO 32 - + H+ + NO 3 - H 2 O (l) + CO 2(g) + Na+ + NO 3 Net ionic: CO 32 - + H+ H 2 O (l) + CO 2(g) 2. 2 KBr(aq) + I 2(g) 2 KI(aq) + Br 2(l) Ionic: K+ + Br- + I 2(g) K+ + I- + Br 2(l) Net ionic: 2 Br- + I 2(g) 2 I- + Br 2(l) 3. Al. Cl 3(aq) + __K 3 PO 4(aq) __Al. PO 4(s) + 3 KCl(aq) Ionic: Al 3+ + Cl- + K+ + PO 43 - Al. PO 4(s) + K+ + Cl. Net ionic: Al 3+ + PO 43 - Al. PO 4(s)

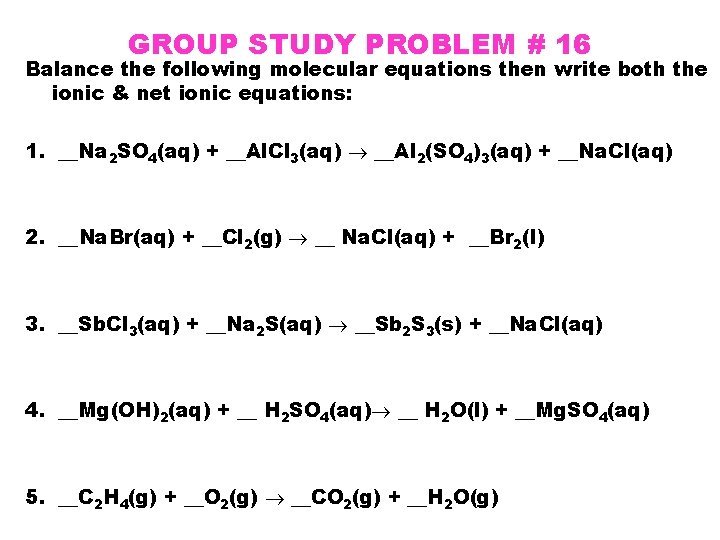
GROUP STUDY PROBLEM # 16 Balance the following molecular equations then write both the ionic & net ionic equations: 1. __Na 2 SO 4(aq) + __Al. Cl 3(aq) __Al 2(SO 4)3(aq) + __Na. Cl(aq) 2. __Na. Br(aq) + __Cl 2(g) __ Na. Cl(aq) + __Br 2(l) 3. __Sb. Cl 3(aq) + __Na 2 S(aq) __Sb 2 S 3(s) + __Na. Cl(aq) 4. __Mg(OH)2(aq) + __ H 2 SO 4(aq) __ H 2 O(l) + __Mg. SO 4(aq) 5. __C 2 H 4(g) + __O 2(g) __CO 2(g) + __H 2 O(g)