Writing Alpha Decay Equations Yesterday you learned that








- Slides: 10

Writing Alpha Decay Equations

• Yesterday you learned that during an ALPHA RADIOACTIVE DECAY, the nucleus of an atoms loses an ALPHA PARTICLE. • The ALPHA PARTICLE consists of: 2 Protons and 2 Neutrons When an atom loses an Alpha Particle the nucleus is changed into another element which has: 1. A MASS NUMBER 4 less than the starting one. 2. An ATOMIC NUMBER 2 less than the starting one.

• If we write an example using the form we learned in class, we place the MASS NUMBER at the TOP LEFT of the CHEMICAL SYMBOL and the ATOMIC NUMBER at the LOWER LEFT of the CHEMICAL SYMBOL like this: • 238 • 92 • To get the new element, we: • 1. Subtracted 4 from the top number and 2 from the bottom number: • 238 – 4 = 234 • 92 – 2 = 90 U

• We then looked at a periodic table and found element number 90, which was Thorium, so we wrote the new element as: • 234 • 90 Th • Now let’s learn to write the complete NUCLEAR EQUATION showing how an ALPHA DECAY occurs. • A NUCLEAR EQUATION is similar to writing a math equation. In a math equation what’s on the left side of the equal sign must equal what’s on the right side of the equal sign.

• A NUCLEAR EQUATION has 4 parts: • Starting Isotope Yield Sign New Isotope + An Alpha Particle • 1. The starting isotope is given in the problem. • 2. In chemistry, a YIELD SIGN is an ARROW and is like an EQUAL SIGN in math. • 3. The new isotope is the one you look up after subtracting 4 and 2 from the original numbers. • 4. The alpha particle symbol is always written as 4 • 2 α

WRITING AN ALPHA EQUATION • Let’s use the U-238 we had earlier and write a nuclear equation for its ALPHA DECAY. • Start by writing the isotope and a yield sign: • 238 • 92 Next, we know we will get an ALPHA PARTICLE so add that to the right of the plus sign: 238 4 92 2 U U α

• Now all that is missing is to add the new isotope we found when we subtracted 4 from the top number (mass number) and 2 from the bottom number (atomic number): • 238 234 4 • 92 90 2 U Th α • And that’s an ALPHA NUCLEAR EQUATION.

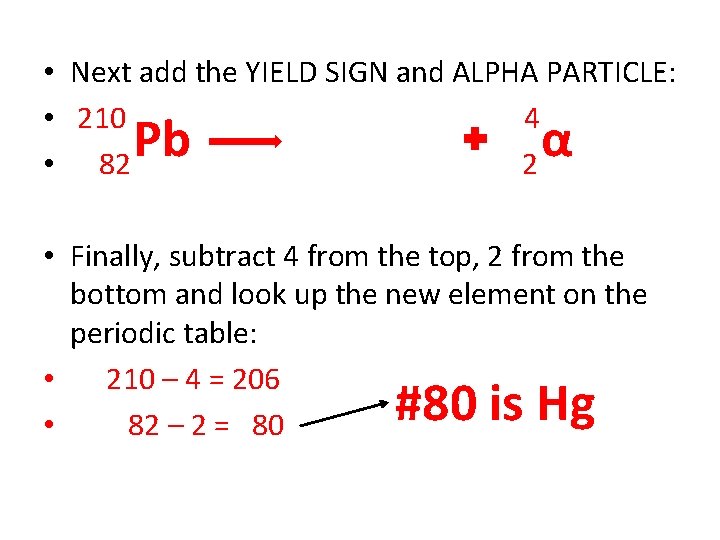
Practice Problem • Write an ALPHA NUCLEAR EQUATION for Pb-210. Looking at the periodic table, we find Pb has an atomic number of 82, so the starting isotope looks like this: 210 82 Pb

• Next add the YIELD SIGN and ALPHA PARTICLE: • 210 4 • 82 2 Pb α • Finally, subtract 4 from the top, 2 from the bottom and look up the new element on the periodic table: • 210 – 4 = 206 • 82 – 2 = 80 #80 is Hg

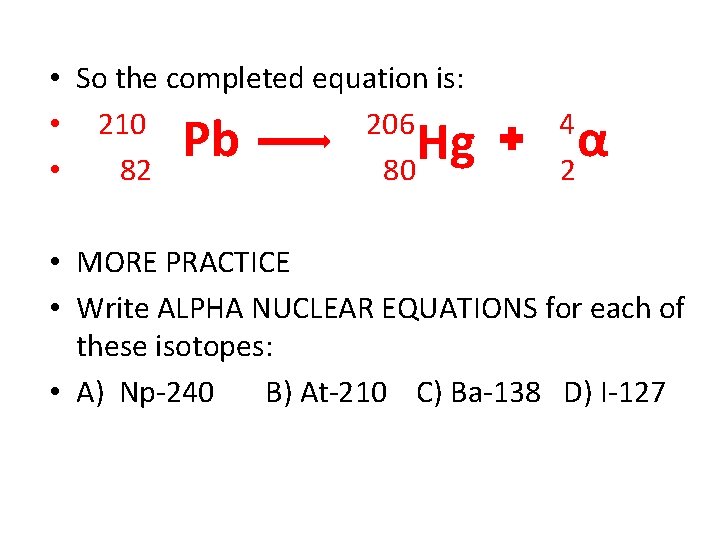
• So the completed equation is: • 210 206 • 82 80 Pb Hg 4 2 α • MORE PRACTICE • Write ALPHA NUCLEAR EQUATIONS for each of these isotopes: • A) Np-240 B) At-210 C) Ba-138 D) I-127