Writing a NIH Grant Application Ellen Pur Ph










- Slides: 12

Writing a NIH Grant Application Ellen Puré, Ph. D, Professor and Associate Vice President of Academic Affairs, Wistar Institute Mitchell Schnall MD, Ph. D, Matthew J. Wilson Professor of Research Radiology 12/11/2009

The NIH 27 institutes and centers Most have active extramural grants programs Under different local leadership and local missions ◦ There are many small differences in operation among the institutes ◦ United by common extramural purpose and language 12/11/2009

The Institute & Centers 12/11/2009

The Institute & Centers 12/11/2009

Types of applications: How are they judged? ¨ K grants (career development) • Trainee, training environment, project ¨ R grants (research projects) • Research project ¨ P grants (multi-investigator grants: PPG, center etc) • Research projects, “the group is stronger than the sum of its parts” 12/11/2009

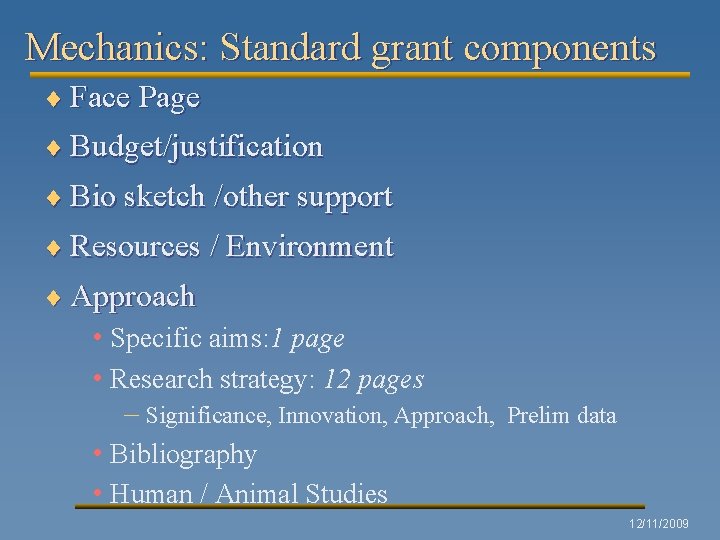
Mechanics: Standard grant components ¨ Face Page ¨ Budget/justification ¨ Bio sketch /other support ¨ Resources / Environment ¨ Approach • Specific aims: 1 page • Research strategy: 12 pages – Significance, Innovation, Approach, Prelim data • Bibliography • Human / Animal Studies 12/11/2009

Strategy assembling application ¨ Identify collaborators early: collect bio/other support ASAP ¨ Complete resources and environment ahead of time (can leverage boiler plate sections if available) ¨ Budget at least 2 weeks before deadline (take a break from the science to complete)-but start at least 3 months in advance! ¨ Details of animal/human studies can go outside of the research strategy (overall description in the research strategy) ¨ Can use appendix for supporting documents, however the Approach section should be self contained. (? not 12/11/2009

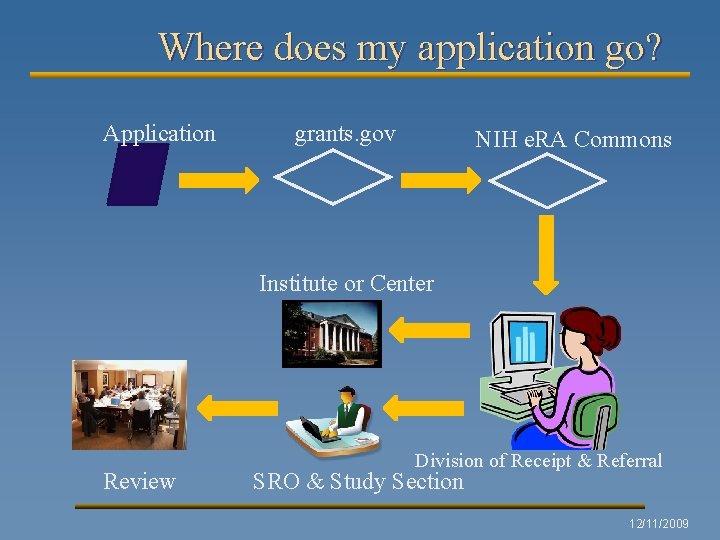
Where does my application go? Application grants. gov NIH e. RA Commons Institute or Center Review Division of Receipt & Referral SRO & Study Section 12/11/2009

¨ What, exactly, is the review process? • The procedure by which each grant application submitted to the NIH receives a fair, independent, expert, and timely evaluation, free from inappropriate influences, so the NIH can support the most promising research. • Two steps: – Peer review panels: generates score /percentile – Institute council review: relevant for large grants/borderline grants 12/11/2009

Review Criteria: R grant Each scored 1 -9 • overall evaluation is not derived from individual components • only overall evaluation is voted by the study section • component scores serve to identify areas of strength/weakness ¨ Significance ¨ Investigator ¨ Innovation ¨ Approach ¨ Environment ¨Overall Evaluation 12/11/2009

Some Statistics ¨ Center for Scientific Review (CSR) • Receives over 80, 000 applications/year • Recruits over 17, 000 external experts ¨ This is the volume of your competition • How do I make my application rise to the top? Grantsmanship! 12/11/2009

Grantsmanship words to live by: ¨ Think first…. . write second!!!! ¨ Vet! ¨ Logic and clarity trumps density • Focus on the “pitch” ¨ A grant is a marketing document (not a clinical trial protocol) ¨ Your primary audience is the review panel ¨ Understand the reviewer’s job, make it easy ¨ Assume a diverse set of reviewers ¨ You share responsibility of a “bad review” 12/11/2009