Write the charges next to the different ions





- Slides: 15

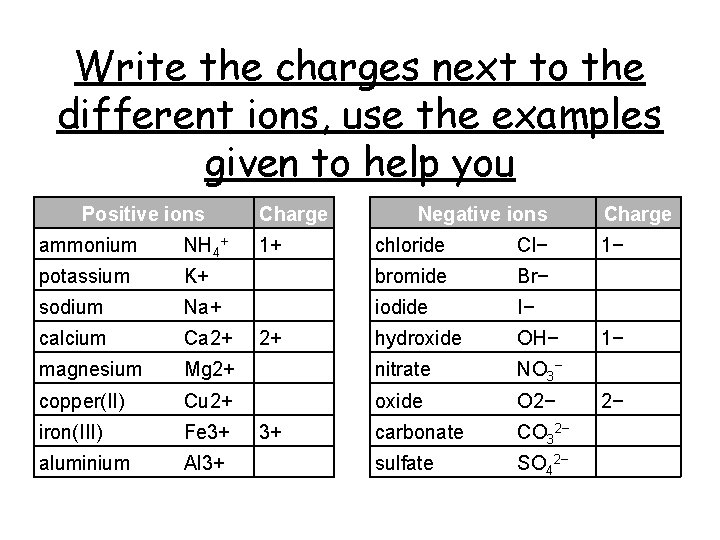
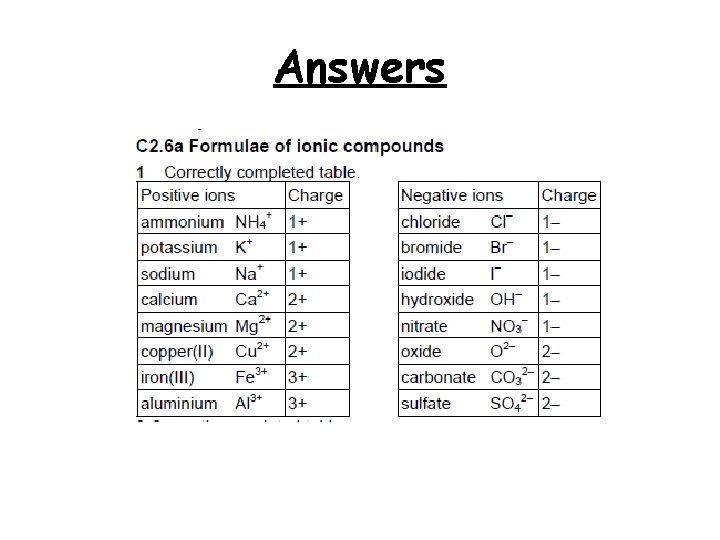
Write the charges next to the different ions, use the examples given to help you Positive ions ammonium NH 4+ potassium Charge 1+ Negative ions chloride Cl− K+ bromide Br− sodium Na+ iodide I− calcium Ca 2+ hydroxide OH− magnesium Mg 2+ nitrate NO 3− copper(II) Cu 2+ oxide O 2− iron(III) Fe 3+ carbonate CO 32− aluminium Al 3+ sulfate SO 42− 2+ 3+ Charge 1− 1− 2−

Answers

Ionic compounds 09 January 2022 Key question: • How are the formulae of ionic Starter: How is the compounds worked out? periodic table useful when finding out the Key words: formula of different • Ionic compounds ions. • Formula • Compound ions Specs: 2. 5 -2. 7

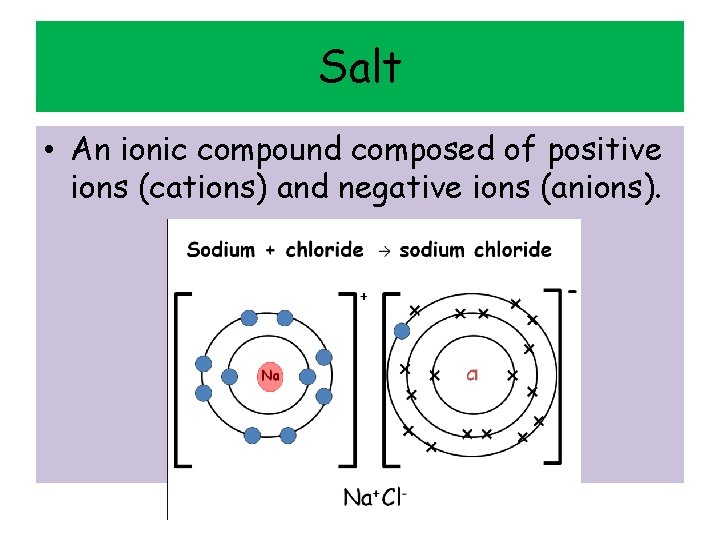
Salt • An ionic compound composed of positive ions (cations) and negative ions (anions).

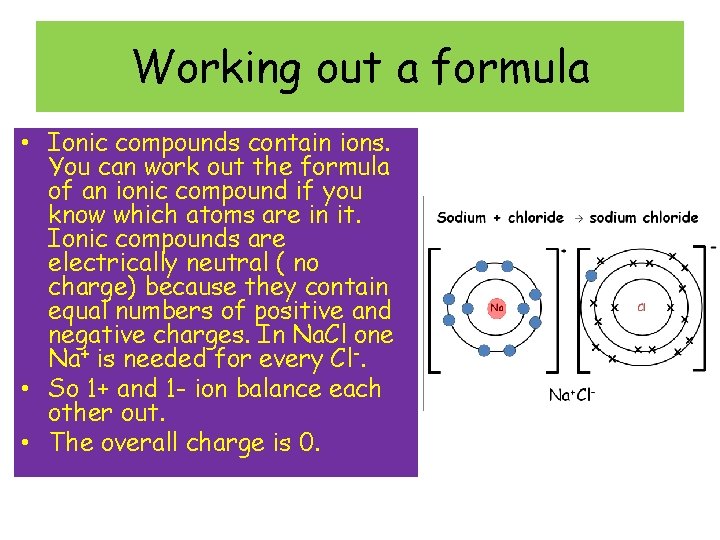
Working out a formula • Ionic compounds contain ions. You can work out the formula of an ionic compound if you know which atoms are in it. Ionic compounds are electrically neutral ( no charge) because they contain equal numbers of positive and negative charges. In Na. Cl one Na+ is needed for every Cl-. • So 1+ and 1 - ion balance each other out. • The overall charge is 0.

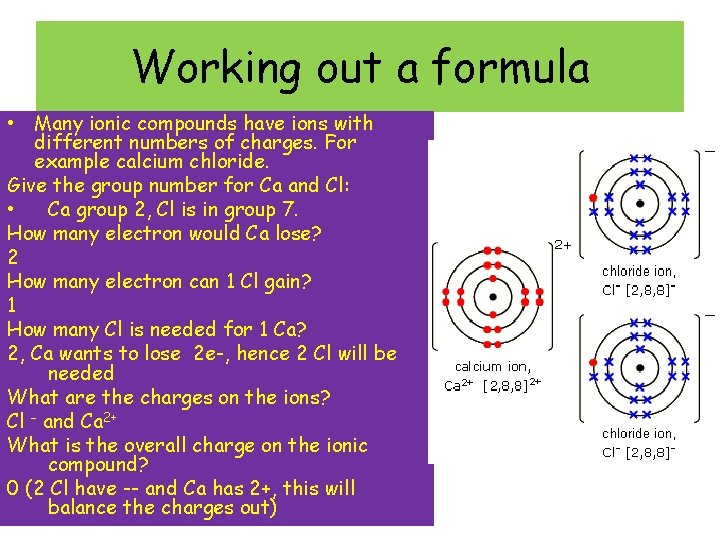
Working out a formula • Many ionic compounds have ions with different numbers of charges. For example calcium chloride. Give the group number for Ca and Cl: • Ca group 2, Cl is in group 7. How many electron would Ca lose? 2 How many electron can 1 Cl gain? 1 How many Cl is needed for 1 Ca? 2, Ca wants to lose 2 e-, hence 2 Cl will be needed What are the charges on the ions? Cl – and Ca 2+ What is the overall charge on the ionic compound? 0 (2 Cl have -- and Ca has 2+, this will balance the charges out)

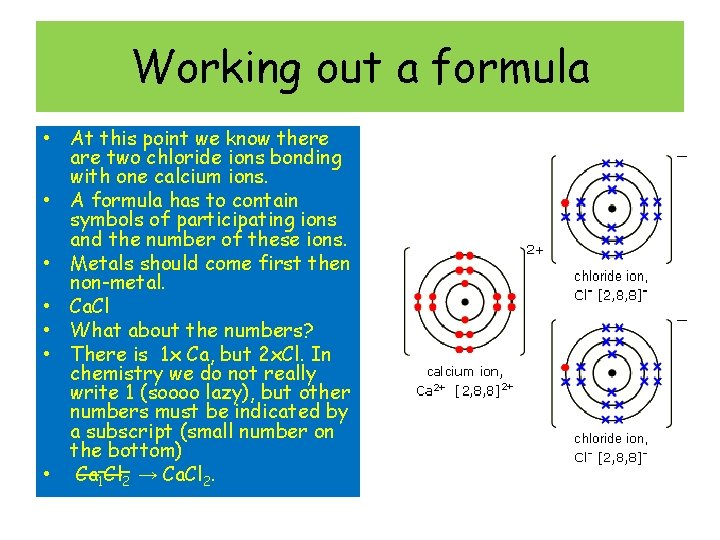
Working out a formula • At this point we know there are two chloride ions bonding with one calcium ions. • A formula has to contain symbols of participating ions and the number of these ions. • Metals should come first then non-metal. • Ca. Cl • What about the numbers? • There is 1 x Ca, but 2 x. Cl. In chemistry we do not really write 1 (soooo lazy), but other numbers must be indicated by a subscript (small number on the bottom) • Ca 1 Cl 2 → Ca. Cl 2.

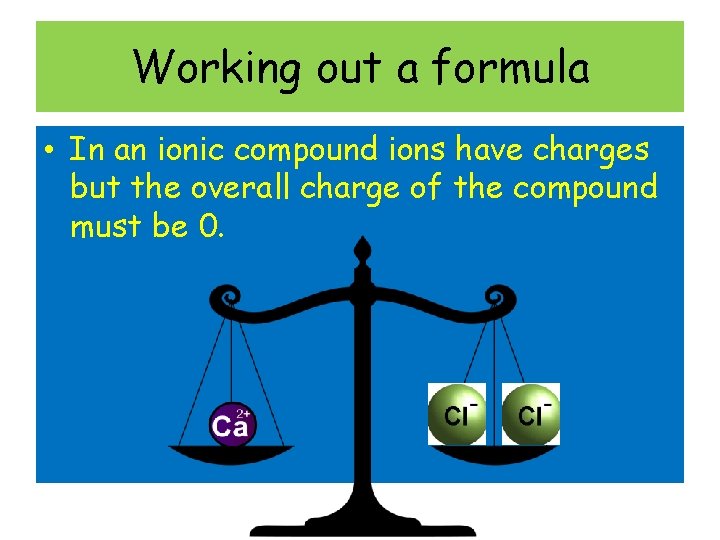
Working out a formula • In an ionic compound ions have charges but the overall charge of the compound must be 0.

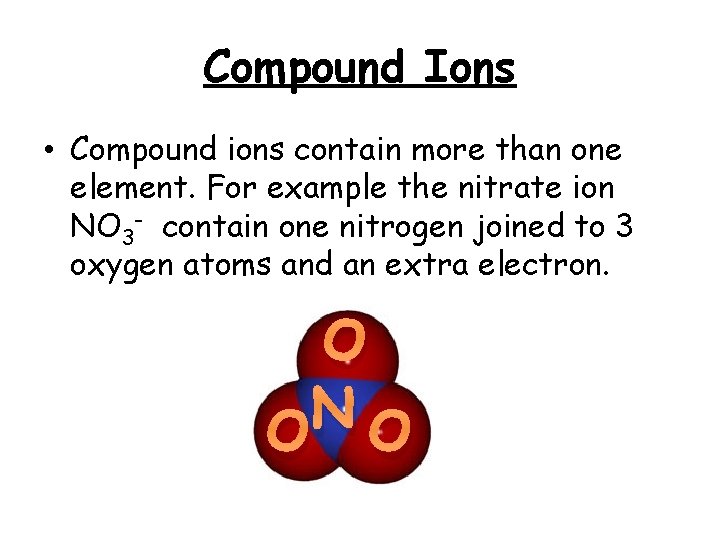
Compound Ions • Compound ions contain more than one element. For example the nitrate ion NO 3 - contain one nitrogen joined to 3 oxygen atoms and an extra electron. O N O O

Common Compound Ions You need to learn these!!! Name Hydroxide Nitrate Carbonate Sulphate Ammonium Symbol OHNO 3 CO 32 SO 42 NH 4+ Charge 11221+

Writing these in a formula • If two or more compound ions of the same type are needed in a formula, the ion must be written inside brackets. For example: Magnesium Nitrate is Mg(NO 3)2 • This shows that the two positive charges on Mg 2+ are balanced by the two negative charges from two NO 3 - ions.

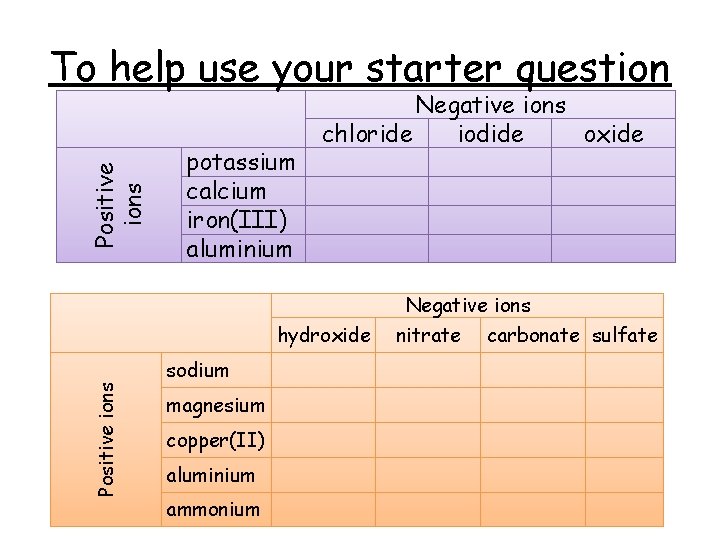
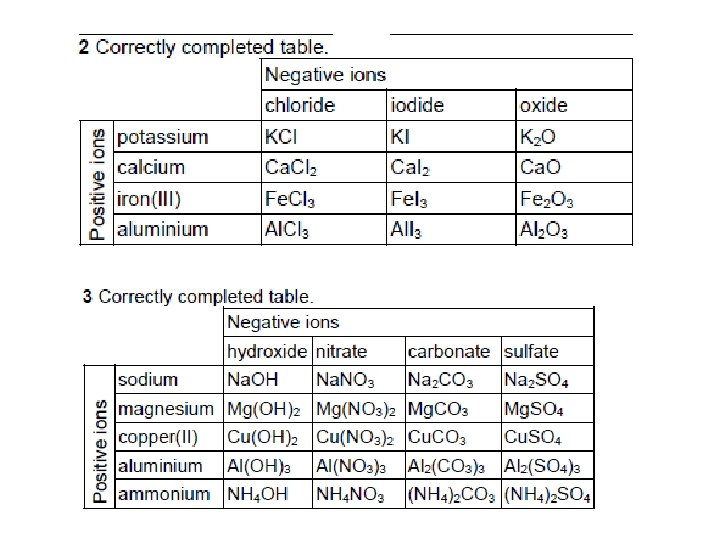
Positive ions To help use your starter question potassium calcium iron(III) aluminium Negative ions chloride iodide oxide hydroxide Positive ions sodium magnesium copper(II) aluminium ammonium Negative ions nitrate carbonate sulfate


What’s in the name? cation + anion -> Ionic Compound • Ionic compounds ending in ‘-ide’- e. g Sodium Chloride • ‘-ide’ ending compounds occur when two different elements combine. • Ionic compounds ending in ‘-ate’- e. g Copper sulphate • ‘-ate’ ending compounds occur when three or more different elements combine and one of them is oxygen. e. g. (Copper Sulphate= copper +sulphur + oxygen) 4

Correctly name the following ionic compounds: a Na. I b Ca. Cl 2 c KOH e Al 2 O 3 g Mg(NO 3)2 h Ca. CO 3 i Na 2 SO 4 a sodium iodide b calcium chloride c potassium hydroxide e aluminium oxide g magnesium nitrate h calcium carbonate i sodium sulfate