Write a Balanced Equation Nitrogen gas reacting with
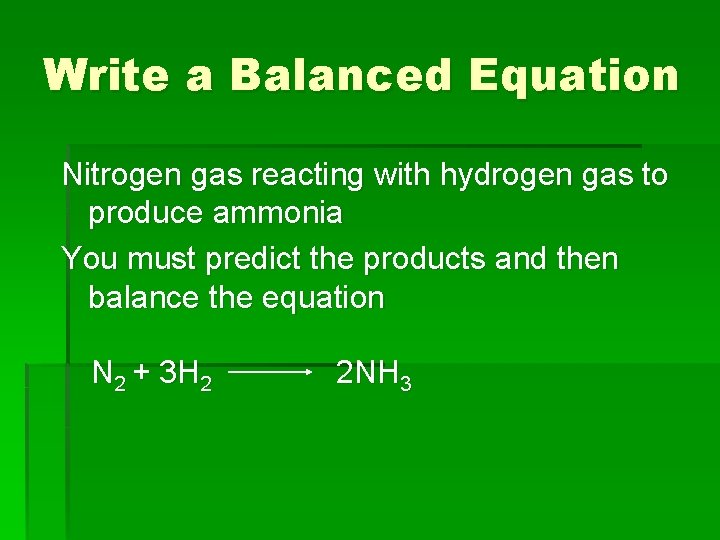
Write a Balanced Equation Nitrogen gas reacting with hydrogen gas to produce ammonia You must predict the products and then balance the equation N 2 + 3 H 2 2 NH 3

Chapter 12 Stoichiometry
What is Stoichiometry? § It is the calculation of the quantitative relationships in chemical equations. § A recipe for chemical equations. § Shows how mass and matter are conserved in reactions

Stoichiometry- things to remember § A chemical equation must be written and it needs to be balanced. § A mole to mole conversion must exist between substances. § ALWAYS CHECK FOR DIATOMIC MOLECULES!!
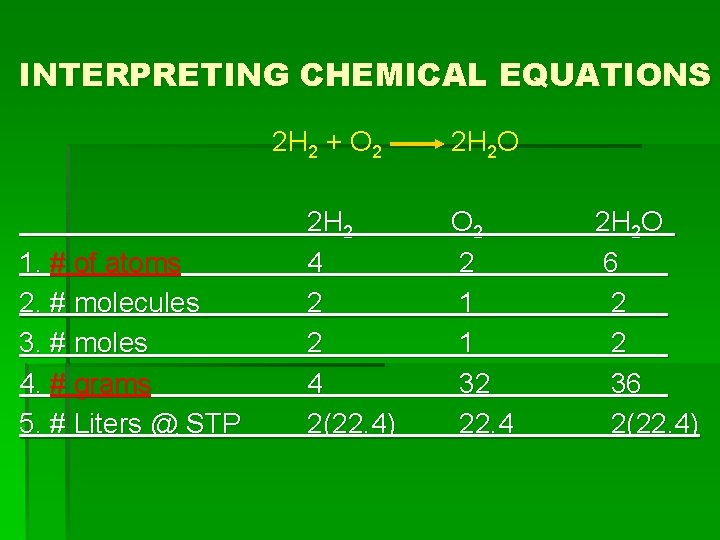
INTERPRETING CHEMICAL EQUATIONS 2 H 2 + O 2 1. # of atoms 2. # molecules 3. # moles 4. # grams 5. # Liters @ STP 2 H 2 4 2(22. 4) 2 H 2 O O 2 2 1 1 32 22. 4 2 H 2 O 6 2 2 36 2(22. 4)
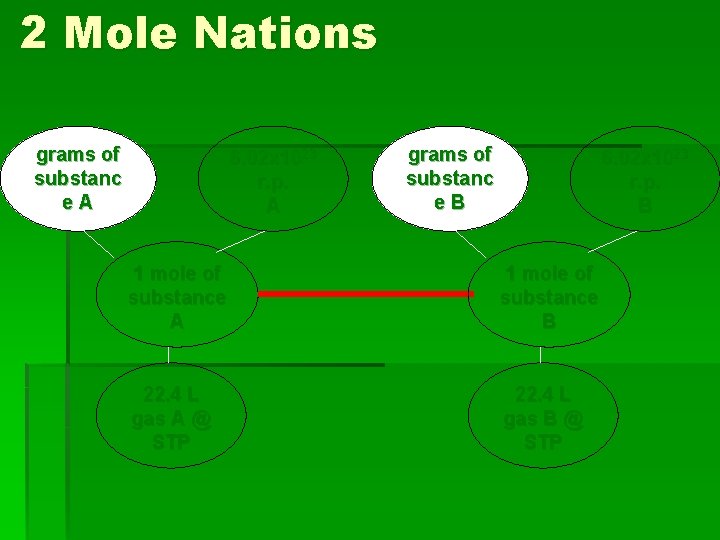
2 Mole Nations grams of substanc e. A 6. 02 x 1023 r. p. A grams of substanc e. B 6. 02 x 1023 r. p. B 1 mole of substance A 1 mole of substance B 22. 4 L gas A @ STP 22. 4 L gas B @ STP
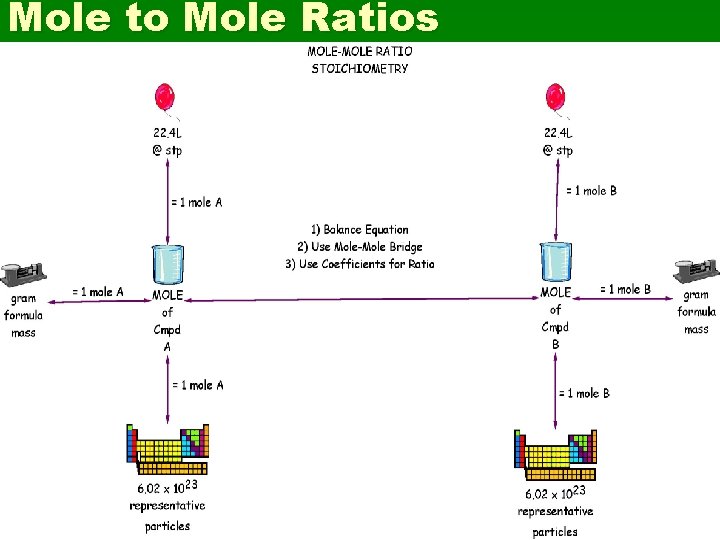
Mole to Mole Ratios
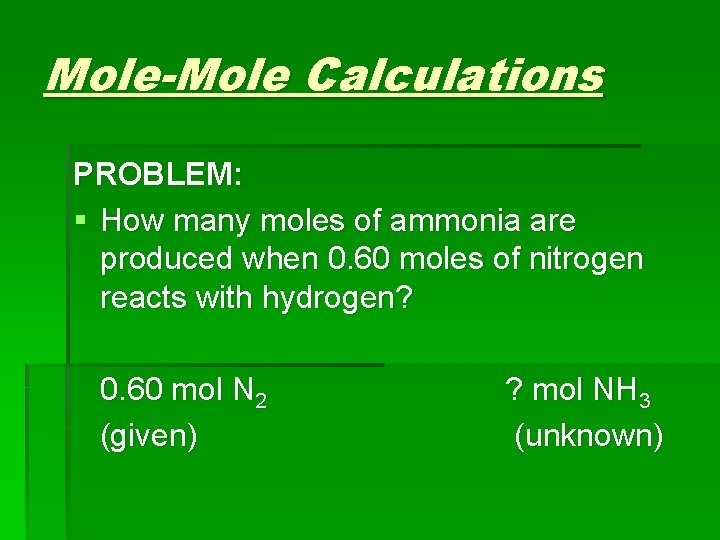
Mole-Mole Calculations PROBLEM: § How many moles of ammonia are produced when 0. 60 moles of nitrogen reacts with hydrogen? 0. 60 mol N 2 (given) ? mol NH 3 (unknown)
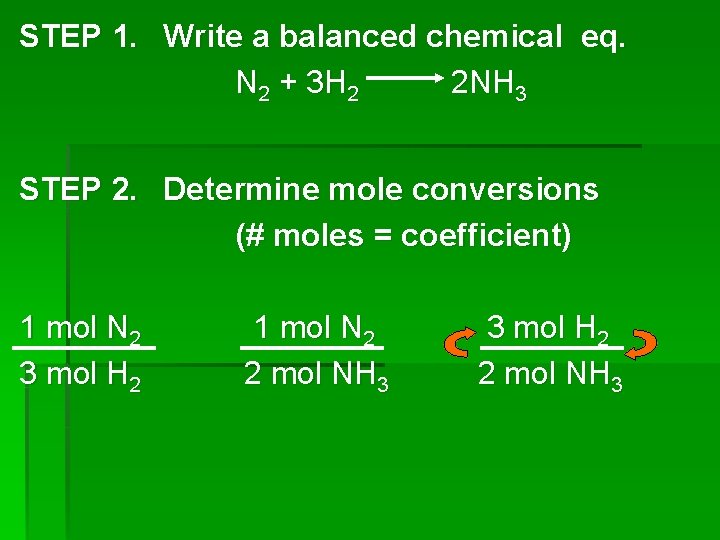
STEP 1. Write a balanced chemical eq. N 2 + 3 H 2 2 NH 3 STEP 2. Determine mole conversions (# moles = coefficient) 1 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 3 mol H 2 2 mol NH 3
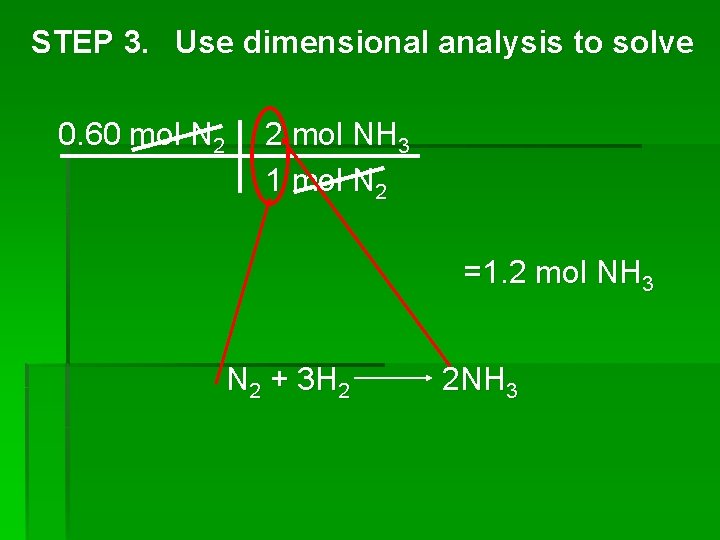
STEP 3. Use dimensional analysis to solve 0. 60 mol N 2 2 mol NH 3 1 mol N 2 =1. 2 mol NH 3 N 2 + 3 H 2 2 NH 3
Mass-Mass Calculations Scientists can’t mass out moles on a balance. Therefore the amount of a substance is typically determined by measuring its mass in grams.
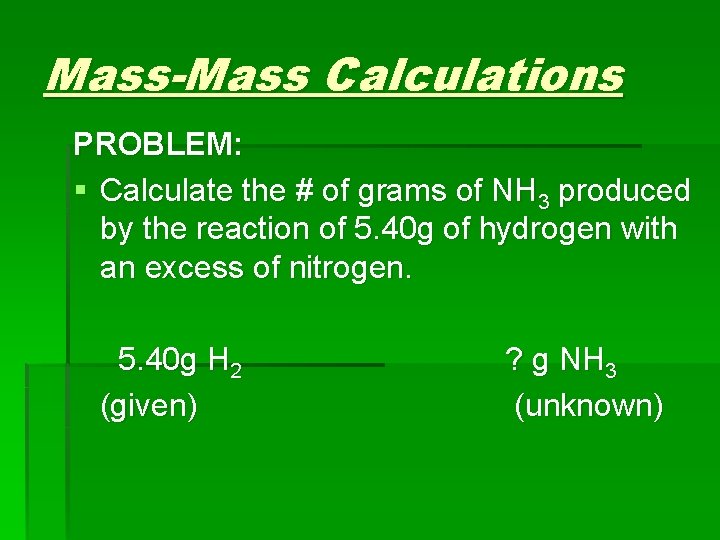
Mass-Mass Calculations PROBLEM: § Calculate the # of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. 5. 40 g H 2 (given) ? g NH 3 (unknown)
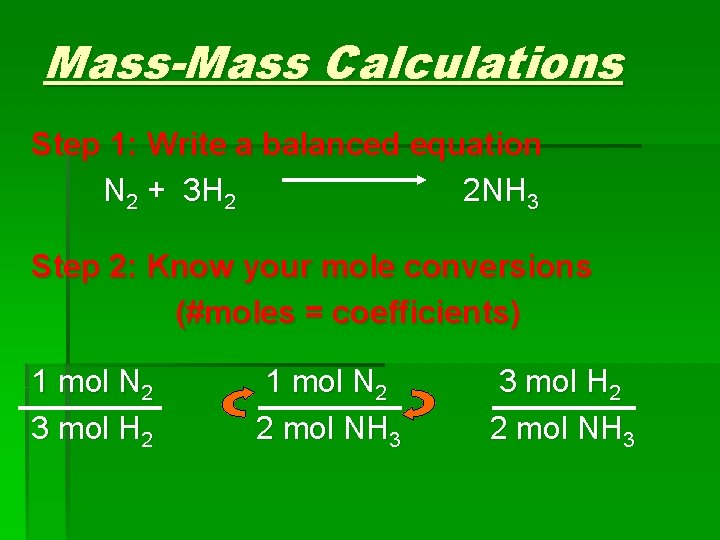
Mass-Mass Calculations Step 1: Write a balanced equation N 2 + 3 H 2 2 NH 3 Step 2: Know your mole conversions (#moles = coefficients) 1 mol N 2 3 mol H 2 1 mol N 2 2 mol NH 3 3 mol H 2 2 mol NH 3
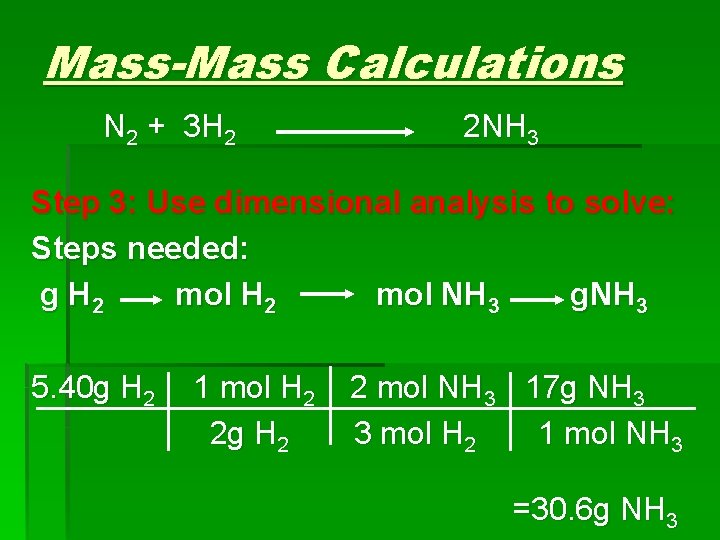
Mass-Mass Calculations N 2 + 3 H 2 2 NH 3 Step 3: Use dimensional analysis to solve: Steps needed: g H 2 mol NH 3 g. NH 3 5. 40 g H 2 1 mol H 2 2 g H 2 2 mol NH 3 17 g NH 3 3 mol H 2 1 mol NH 3 =30. 6 g NH 3
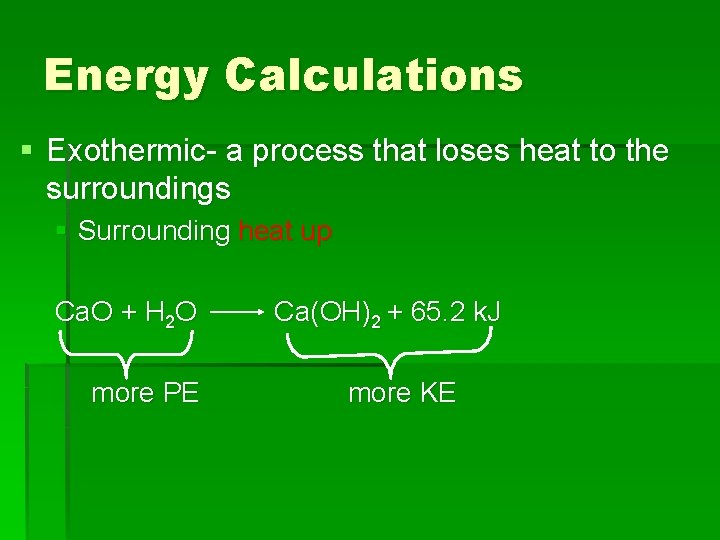
Energy Calculations § Exothermic- a process that loses heat to the surroundings § Surrounding heat up Ca. O + H 2 O more PE Ca(OH)2 + 65. 2 k. J more KE
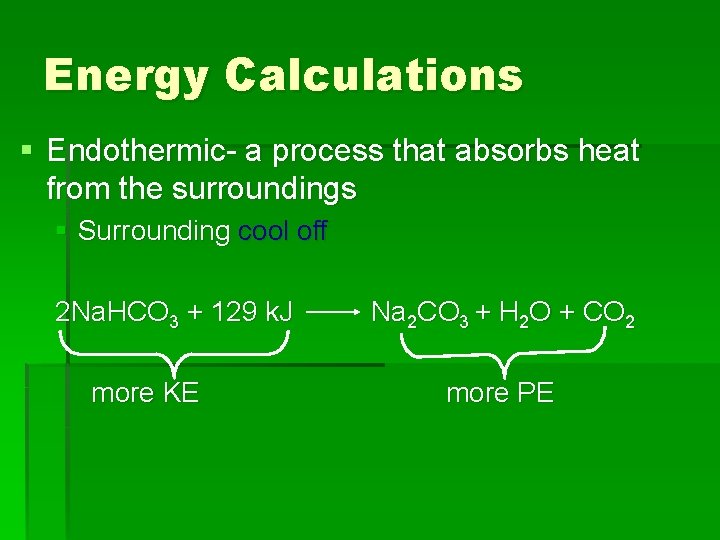
Energy Calculations § Endothermic- a process that absorbs heat from the surroundings § Surrounding cool off 2 Na. HCO 3 + 129 k. J more KE Na 2 CO 3 + H 2 O + CO 2 more PE
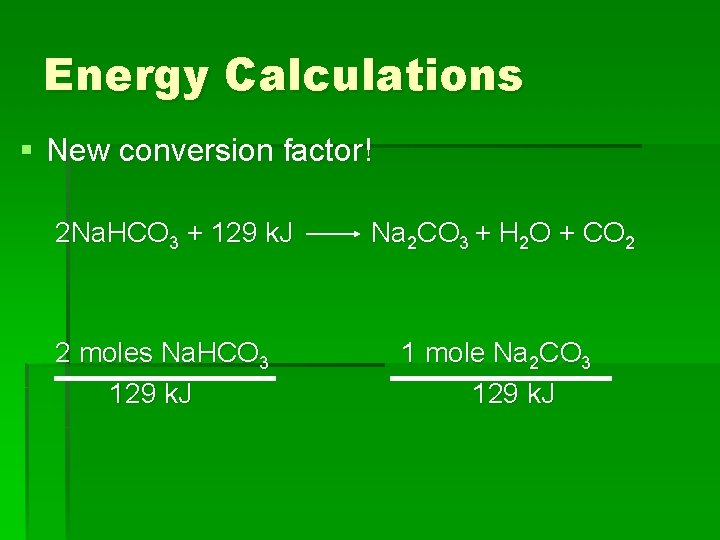
Energy Calculations § New conversion factor! 2 Na. HCO 3 + 129 k. J 2 moles Na. HCO 3 129 k. J Na 2 CO 3 + H 2 O + CO 2 1 mole Na 2 CO 3 129 k. J
- Slides: 17