WORLD of MACROMOLECULES CARBOHYDRATES 1 Composed of carbon
WORLD of MACROMOLECULES
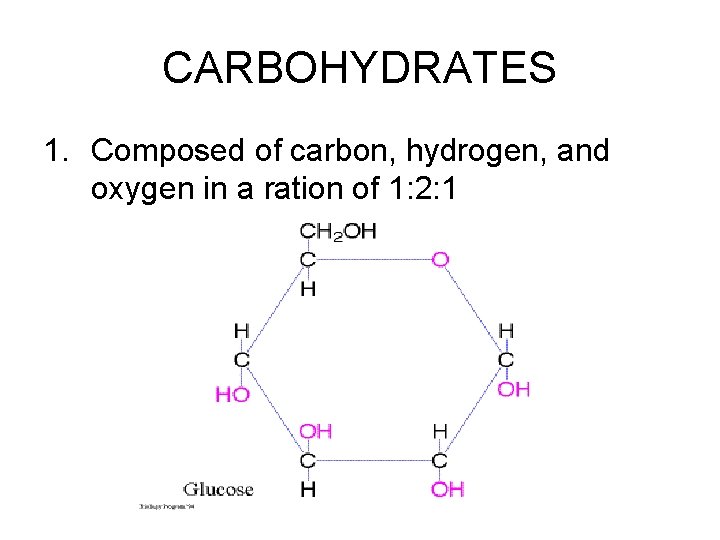
CARBOHYDRATES 1. Composed of carbon, hydrogen, and oxygen in a ration of 1: 2: 1
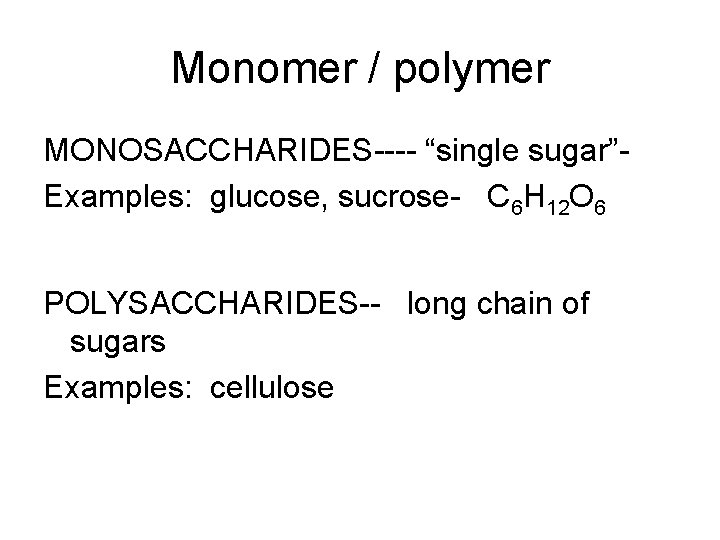
Monomer / polymer MONOSACCHARIDES---- “single sugar”Examples: glucose, sucrose- C 6 H 12 O 6 POLYSACCHARIDES-- long chain of sugars Examples: cellulose
ISOMERS Molecules with the same chemical formula but DIFFERENT SHAPE AND STRUCTURE
LIPIDS • Composed of mostly long chains of CARBON and HYDROGEN with a CARBOXYL (COOH) at one end. Examples- triglycerides, phospholipids

LIPIDS HYDROPHILIC- “water loving”- POLARCarboxyl end HYDROPHOBIC- “water fearing” – NONPOLAR- fatty acid tail
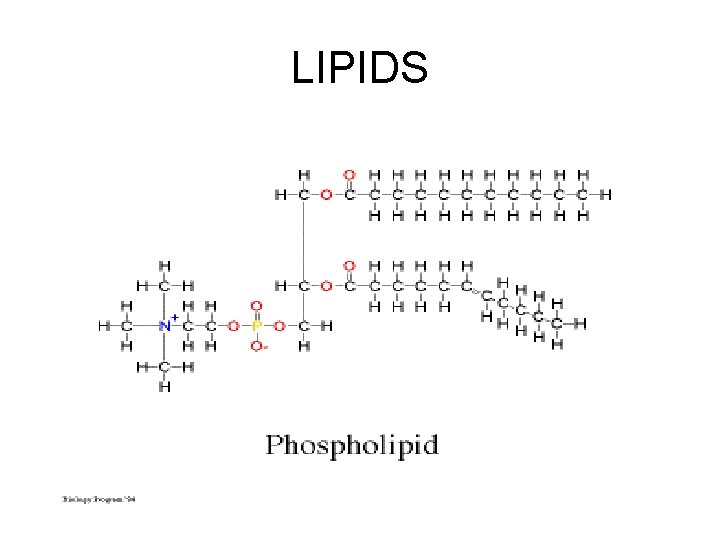
LIPIDS

TYPES OF LIPIDS SATURATED- “bad animal fats”- contains no double bonded carbon atoms UNSATURATED- “ good plant fats”contains some double bonded carbon atoms
PROTEINS Composed of carbon, hydrogen, oxygen, and NITROGEN There are 20 different amino acids each contain an AMINO group (NH 2) and a CARBOXYL group (COOH)

PROTEINS R GROUP – varies among amino acids and gives different proteins very different shapes Different shapes of proteins allow them to perform different roles.
Monomer / polymer AMINO ACID- PROTEIN-
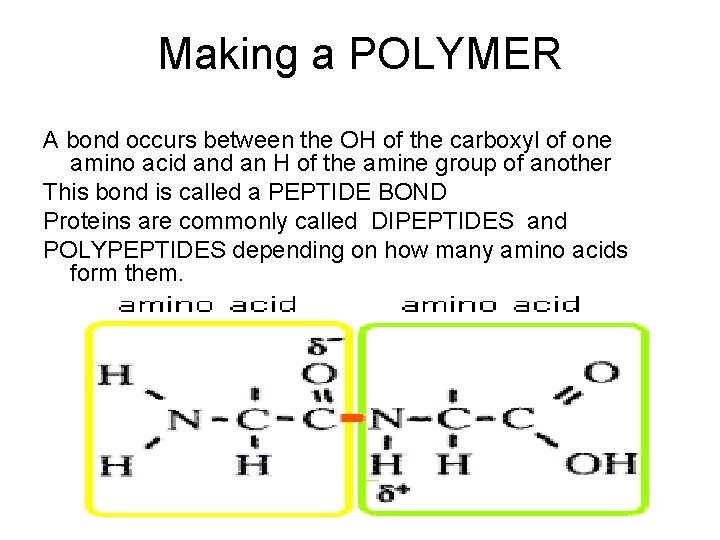
Making a POLYMER A bond occurs between the OH of the carboxyl of one amino acid an H of the amine group of another This bond is called a PEPTIDE BOND Proteins are commonly called DIPEPTIDES and POLYPEPTIDES depending on how many amino acids form them.

USES OF PROTEIN -Used to make skin and muscle in animals - Most importantly used as a CATALYST in all living things called ENZYMES
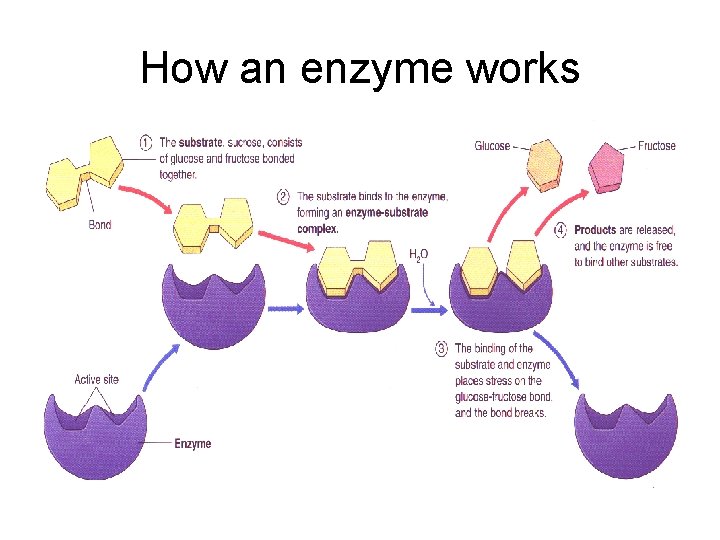
How an enzyme works
NUCLEIC ACIDS Composed of carbon, hydrogen, oxygen, and NITROGEN and PHOSPHOROUS Examples: RNA and DNA Consists of 1. Phosphate group 2. Five carbon sugar 3. Nitrogen base
Monomer / polymer NUCLEOTIDE- NUCLEIC ACID-
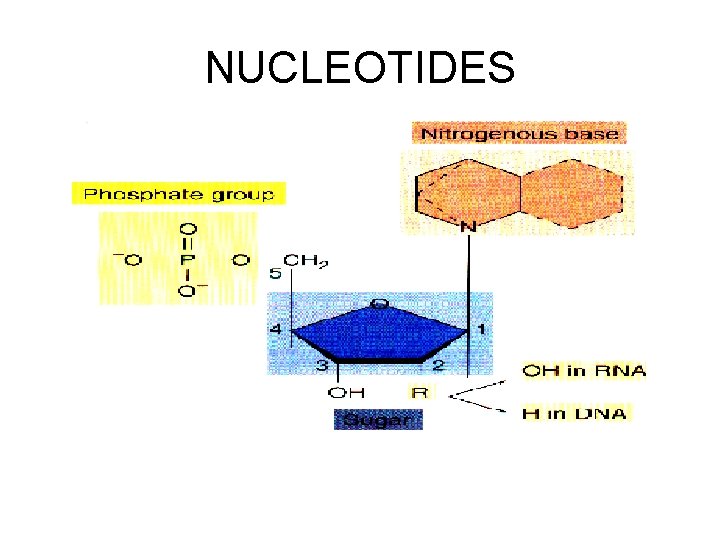
NUCLEOTIDES
- Slides: 17