Workshop on the Veterinary Products for Asian countries















![Contents of Form 5 -A FORM 5 -A [See rule 26 (1)] APPLICATION FORM Contents of Form 5 -A FORM 5 -A [See rule 26 (1)] APPLICATION FORM](https://slidetodoc.com/presentation_image/2c112c63c704c33cb858804c00894430/image-17.jpg)




























- Slides: 45

Workshop on the Veterinary Products for Asian countries, 2010 22~ 26 November, 2010 National Veterinary Research and Quarantine Service (NVRQS) Republic of Korea organized by NVRQS and KAHPA. By: ABDULLAH DIYO DEPUTY DRUGS CONTROLLER (REG) MINISTRY OF HEALTH GOVERNMENT OF PAKISTAN ISLAMABAD BY: ABDULLAH ABRO MOH PAK 1

A brief Introduction of Pakistan The Islamic republic of Pakistan emerged as an independent sovereign state on 14 th August 1947, as a result of the division of former British India. It lies between 23 -35 to 37 - 05 north latitude and 60 -50 to 77 - 50 east longitude touching the Hindukush Mountains in the north and extending from the Pamirs to the Arabian Sea. Pakistan covers 796, 095 sq. km with a population of 132. 35 million according to population census 1998. BY: ABDULLAH ABRO MOH PAK 2

A brief Introduction of Pakistan It is divided into four provinces: Sindh, Punjab, Khyber Pakhtun Khwa Balochistan. Climatically, Pakistan enjoys a considerable measure of variety. North and north western high mountainous ranges are extremely cold in winter while the summer months of April to September are very pleasant BY: ABDULLAH ABRO MOH PAK 3

A brief Introduction about Pakistan The country has an agricultural economy with a network of canals irrigating a major part of its cultivated land. Wheat, cotton, rice, millet and sugar cane are the major crops. Among fruits: mangos, oranges, bananas and apples are grown in abundance in different parts of the country. The main natural resources are natural gas, coal, salt and iron. The country has an expanding industry. Cotton, Textiles, sugar, cement, and chemicals play an important role in its economy. The country comprises of a vast area that was the great center of ancient civilizations of the world. Its historical sites beginning with stone-age to Twentieth Century A. D are a mirror of the life of its people who were, by nature, simple, virile, hospitable and hard working. Ancient sites excavated in Taxila, Harappa, and Moenjodaro speak volumes for Pakistan’s rich cultural background dating back to 3, 000 B. C. BY: ABDULLAH ABRO MOH PAK 4

An Introduction of Ministry of Health The Ministry of Health is responsible for matters concerning National Planning and Coordination in the field of Health. International Liaison, legislation pertaining to the drugs and medicines, administration of drugs Act 1976. Among major nursing, dental, pharmaceutical, Para-medical and allied subject such as maintenance of educational standard, education abroad, educational facilities for backward areas and foreign nationals except the nomination of candidates from the FATA for admission to Medical Colleges. Ministry of Health consists of one division; Health Division. BY: ABDULLAH ABRO MOH PAK 5

Drugs Control Organization works under Ministry of Health and it is one of the main division of Mo. H. It functions mainly as Secretariat of the Drugs Act, 1976. The Drugs Act, 1976 comprises Federal and Provincial subjects. The Federal Govt. regulates manufacture, registration, pricing, import and export of drugs. BY: ABDULLAH ABRO MOH PAK 6

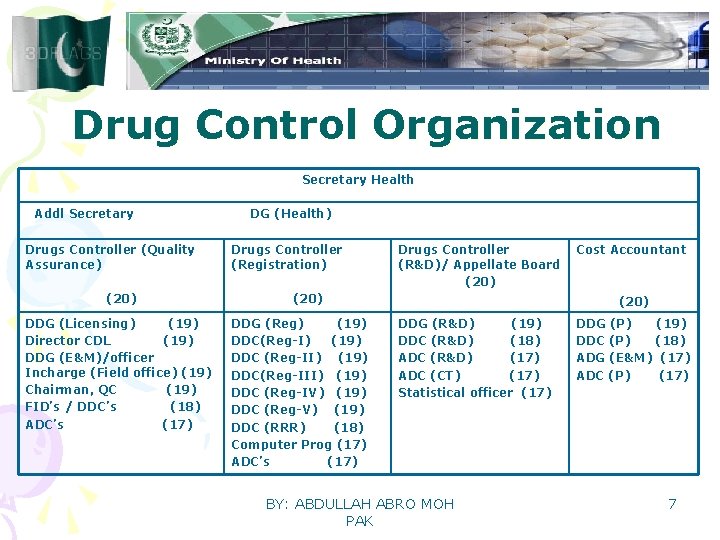
Drug Control Organization Secretary Health Addl Secretary Drugs Controller (Quality Assurance) (20) DDG (Licensing) (19) Director CDL (19) DDG (E&M)/officer Incharge (Field office) (19) Chairman, QC (19) FID’s / DDC’s (18) ADC’s (17) DG (Health) Drugs Controller (Registration) Drugs Controller (R&D)/ Appellate Board (20) DDG (Reg) (19) DDC(Reg-I) (19) DDC (Reg-II) (19) DDC(Reg-III) (19) DDC (Reg-IV) (19) DDC (Reg-V) (19) DDC (RRR) (18) Computer Prog (17) ADC’s (17) Cost Accountant (20) DDG (R&D) (19) DDC (R&D) (18) ADC (R&D) (17) ADC (CT) (17) Statistical officer (17) BY: ABDULLAH ABRO MOH PAK DDG (P) (19) DDC (P) (18) ADG (E&M) (17) ADC (P) (17) 7

LEGISLATION Drugs Act, 1976 Regulates import, export, manufacture, storage, distribution and sale and pricing of drugs Rules are framed to regulate licencing, registration, advertising, labelling, packaging, import and export of drugs. BY: ABDULLAH ABRO MOH PAK 8

FUNCTIONS Federal Government Manufacture (Licensing) Registration Pricing Import Export Provincial Government Sale Storage Distribution Expiry of Drugs BY: ABDULLAH ABRO MOH PAK 9

CENTRAL LICENSING BOARD • Main Functions Under Drugs Act 1976: GMP Compliance, Licensing and Renewal of Pharma Units • Composition Headed by Additional Secretary 13 members including • Technical Experts (Directors Drug Testing Laboratories, Professor of Pharmacy, Pharmaceutical Production and Quality Control) • Representatives from Ministry of Law and Justice Division. • Observers BY: ABDULLAH ABRO MOH PAK 10

LICENSING OF PHARMACEUTICAL UNITS • Types of Licenses – Basic Manufacture – Semi Basic Manufacture – Formulation – Repacking – Experimental • Licensing Activities Site Verification for Suitability Layout plan approval Inspection for approval for Licensing GMP Inspections BY: ABDULLAH ABRO MOH PAK 11

DRUGS REGISTRATION BAORD • Main Functions Under Drugs Act 1976: Registration of Drugs Renewal of Registrations • Composition Headed by Director General Health 14 members including • Technical Experts (Clinical Physician, Clinical Pharmacologist, Professor of Pharmacy, Animal Husbandry Commissioner, Pharmaceutical Production and Quality Control, Biologicals) • Representatives from Ministry of Law and Justice Division. IPO • Observers BY: ABDULLAH ABRO MOH PAK 12

Technical Committees • Technical committees working under Registration Board – Expert Committee on Biological Drugs – Expert Committee on Veterinary Drugs • Expert Pool for new drug evaluation – – Medical Expert Biological Expert Pharmaceutical Expert Veterinary Expert BY: ABDULLAH ABRO MOH PAK 13

DRUG REGISTRATION • Criteria for Registration – Efficacy – Safety – Quality • Type of Registration – Registration of Locally Manufactured Drugs – Registration of Imported Drugs BY: ABDULLAH ABRO MOH PAK 14

DRUG REGISTRATION PROCESS • Receiving of application as per Form along with Fee. • Primary scrutiny of application in Reg-I section. • Referred to ECVD or ECBD accordingly. • The recommendation of above committees are considered by DRB. • Inspection of manufacturer abroad for GMP audit and local facilities accordingly. • DRB finally approves the registration of drugs after completion of above formalities. • Finally Registration Letter is issued • Presently prices of Vet drugs are decontrolled. BY: ABDULLAH ABRO MOH PAK 15

Forms for Registration FORM 5 : Application for registration of a drug for local manufacture FORM 5 -A : Application form for registration of an imported drug FORM 5 -B : Application form for renewal of registration of all kinds of drugs FORM 5 -D : Application form for registration of a dosage form containing a new drug molecule or a new combination / dosage form, for local manufacture FORM 5 -E : Application form for the registration to manufacture a patented drug BY: ABDULLAH ABRO MOH PAK 16
![Contents of Form 5 A FORM 5 A See rule 26 1 APPLICATION FORM Contents of Form 5 -A FORM 5 -A [See rule 26 (1)] APPLICATION FORM](https://slidetodoc.com/presentation_image/2c112c63c704c33cb858804c00894430/image-17.jpg)
Contents of Form 5 -A FORM 5 -A [See rule 26 (1)] APPLICATION FORM FOR REGISTRATION OF AN IMPORTED DRUG I / We …………………. . . . of …………………………… hereby apply for registration of the drug, namely …………………… details of which are enclosed. Date ………………. Signed ……………… Place ………………. BY: ABDULLAH ABRO MOH PAK 17

ENCLOSURES OF THE APPLICATION FOR REGISTRATION OF AN IMPORTED DRUG Dosage Form: ------1. Name and address of the indentor or agent. 2. Name and address of manufacturer of the drug. 3. Brand (Proprietary) name of the drug. 4. The chemical name(s) and , as appropriate and available, the established (generic) and synonyms of the drug. 5. Strength of active ingredient(s) per unit, e. g. , each tablet or 5 ml, etc. contains. BY: ABDULLAH ABRO MOH PAK 18

6. 7. 8. 9. 10. 11. 12. 13. Country from where the drug is proposed to be imported. 7 - The names of the countries, other than Pakistan, wherever the drug is registered and sold. Specify the brand name(s), if other than the brand name applied for. (Free sale certificate of country of import to be attached. ) Pharmacological group. Proposed route of administration. Composition (actives & excepients) including statement of the quantitative composition, giving the weight or measure for each active substance used in the manufacture of the dosage form. Recommended clinical use. Out line of method of manufacture. A full description of the specifications and analytical methods necessary to assure the identity, strength, quality, purity and homogeneity through out the shelf life of the drug product. BY: ABDULLAH ABRO MOH PAK 19

14. Labeling and prescribing information (to be mentioned on the pack/leaflet) specimen or draft shall be submitted. 15. Proposed dosage. 16. Proposed shelf life of the drug. 17. Unit price of the drug, e. g. per tablet, per capsule, per 5 ml, etc. 18. Proposed storage conditions of the finished product. 19. Persons under whose direct supervision and control the drug applied for registration shall be manufactured with the following details, namely: a. total number of technical staff; and b. name, qualification and designation of the persons directly supervising the manufacture of the drug, and any change shall be properly documented and recorded and maintained by the manufacturer. BY: ABDULLAH ABRO MOH PAK 20

20. Name of equipments that will be used in the manufacture of the applied drug: BY: ABDULLAH ABRO MOH PAK 21

21. Production capacity of the manufacturer per shift for the drug applied. 22. Name, qualification and designation of the persons who will be responsible for the quality control of the drug. 23. Description of the equipment to be used for the quality control of the active raw material and the finished products. 24. Facility of the water processing, with specifications. 25. Environment control processing with details. 26. Attach the last Inspection Report conducted by the concerned Regulatory Authorities. 27. Clinical data (along with data of clinical trials conducted and safety data of the drug, with reported side effects and adverse drug reactions in the indigenous community). 28. Clinical justification. 29. Dosage form stability profile. 30. Any other relevant information that may be required by the Board. BY: ABDULLAH ABRO MOH PAK 22

UNDERTAKING I / We hereby undertake that the above given information is true and correct to the best of my / our knowledge and belief. Signature of the authorized importer BY: ABDULLAH ABRO MOH PAK 23

CHECK LIST FOR REGISTRATION OF IMPORTED DRUGS 1. Application on the prescribed form under SRO. 662 (I)/2005 is required. Each page should be duly signed and stamped by the applicant and responsible technical personal of the manufacturer along with page numbering and indexation in the order of checklist. 2. Electronic copy (CD which should be read only) of the application and relevant enclosures of application. 3. Name and complete address of the applicant. 4. Name and complete address of manufacturer abroad. 5. (a) Brand (Proprietary) name of drug in the country or origin. (b) Generic/International non-proprietary name. 6. Strength of active ingredient(s) per unit, e. g. , each tablet or 5 ml, etc. contains. Also provide label claim. 7. Country from where the drug is proposed to be imported. BY: ABDULLAH ABRO MOH PAK 24

CHECK LIST FOR REGISTRATION OF IMPORTED DRUGS 8. The names of the countries, other than Pakistan, wherever the drug is registered and sold. Provide the documentary proof. 9. Pharmacological group. 10. Proposed route of administration. 11. Composition (actives & excepients) including statement of the quantitative composition, giving the weight or measure for each active substance used in the manufacture of the dosage form. Also provide the label claim. 12. Recommended clinical use along with documentary evidence of the approved indications in the country of origin and in developed countries. BY: ABDULLAH ABRO MOH PAK 25

13. Out line of method of manufacture. Complete manufacturing operations from step A to Z in addition to following. a). Master formula b). Manufacturing operation c). Critical steps identified which may change the results d). In process quality control e). Validation of equipments and manufacturing methods etc. 14. A full description of the specifications and analytical methods necessary to assure the identity, strength, quality, purity and homogeneity through out the shelf life of the drug product. Provide complete specification of raw material (both active and non active) and finished drugs for assuring the following: a). Identity of the product b). Strength c). Quality d). Purity e). Homogenacity Protocols of test applied, limits for qualification and its validation is required. (For pharmacopoeial drugs, copies of pharmacopoeial reference of the finished drugs shall be enclosed with the 26 BY: ABDULLAH ABRO MOH application). PAK

15. Labeling and prescribing information (to be mentioned on the pack/leaflet) specimen or draft shall be submitted. a). Leaflet information Recommended clinical uses, contraindications, side effects, precautions, drug interaction, toxicity, dosage, composition and any other information for the safe and effective use of drug. b). Specimen label and carton as per Drug Labeling Rule 1986. Two packs of finished samples shall also be provided. 16. Proposed dosage of the drug (Adults, Children by age group, Infants, Special groups). BY: ABDULLAH ABRO MOH PAK 27

17. Proposed shelf life of the drug. Stability studies including accelerated stability studies in extreme conditions, shelf life, expiry date and storage conditions. 18. Unit price of the drug, e. g, per tablet, per capsule, per 5 ml, including pack price of the drug etc. 19. Proposed storage conditions of the finished product. The storage condition shall be derived from the stability studies. 20. Persons under whose direct supervision and control the drug applied for registration shall be manufactured with the following details, namely: (a) Total number of technical staff; and (b) Name, qualification and designation of the persons directly supervising the manufacture of the drug, and any change shall be properly documented and recorded and maintained by the manufacturer. BY: ABDULLAH ABRO MOH PAK 28

21. Name of equipments that will be used in the manufacture of the applied drug: 22. Production capacity of the manufacturer per shift for the drug applied. 23. Name, qualification and designation of the persons who will be responsible for the quality control of the drug. 24. Description of the equipment to be used for the quality control of the active raw material and the finished products. 25. Facility of the water processing, with specifications. BY: ABDULLAH ABRO MOH PAK 29

26. Environment control processing with details including following: a). cleaning validation b). HVAC system c). Maintenance of clean area. 27. Attach the last Inspection Report conducted by the concerned Regulatory Authorities. Inspection report shall be translated in English. 28. Clinical data (along with data of clinical trials conducted and safety data of the drug, with reported side effects and adverse drug reactions in the indigenous community). Data should be specific to the brand not the molecule. Data include phase I to IV clinical trials. 29. Clinical justification including risk/ benefit ratio, therapeutic superiority over the existing therapy available and cost effectiveness. 30. Dosage form stability profile. BY: ABDULLAH ABRO MOH PAK 30

31. Free Sale Certificate & G. M. P. certificate (in original) from the regulatory authority in the country of origin as per approved format of the W. H. O. 32. Evidence of Free Sale of applied molecule in any one of the EU countries, USA, Japan and Australia. 33. Sole Agency Agreement with the manufacturer / authorized agent abroad. List of product shall also be attached for which the Sole Agency is given. 34. Information as per guidelines for the registration of biological drugs up loaded on the official website www. dcomoh. gov. pk. (For the application of biological drugs). 35. Original Credentials of the company duly endorsed by the Pakistan Embassy/Consulate office in the country of export. 36. International price comparison (for New Drug) 37. Research papers published in internationally recognized journals. (For New Drug) BY: ABDULLAH ABRO MOH PAK 31

38. Treasury Challan of Rs. 15000/- (in original) being the registration fee to be deposited in Federal Government treasury under the head of account: C-Non Tax Revenue C 02 -Receipts from Civil Administration and other Functions C 028 -Social Services C 02841 -Health-Other Receipts 39. Four copies of registration dossier/technical data/literature of the drug for Expert Opinion (for new drug). 40. Copy of Drug Sale License. 41. Undertaken by the applicant that same generic having same composition of active ingredient is not already registered with the applicant. Please ensure that the above referred documents/ information complete in all respects, are positively supplied with the registration application. In case of incomplete application, it will be returned to the applicant and considered as disposed off. 32 BY: ABDULLAH ABRO MOH PAK

DETAILS OF REGISTERED DRUGS • HUMAN DRUGS Imported Human Drugs………………. . 6250 Locally Manufactured Human Drugs………. . … 38000 Total………………. . . 44, 250 • VETERINARY DRUGS Imported Veterinary Drugs……………… 2528 Local Veterinary Drugs…………. . 3542 Total………………………. 6, 070 No of Total Drugs Registered in Pakistan……. . . 50, 320 Information submitted on 21 -01 -2010 BY: ABDULLAH ABRO MOH PAK 33

Total National & Multinational Units in Pakistan Province Punjab Sindh KPK Balochistan Azad Kashmir Total National Multinational Total Units 269 117 77 07 04 474 06 20 02 28 275 137 77 09 04 502 BY: ABDULLAH ABRO MOH PAK 34

National Veterinary Units in Pakistan Province National Punjab Sindh KPK Balochistan Azad Kashmir Total BY: ABDULLAH ABRO MOH PAK 37 10 03 02 52 35

REGULATORY AND MONITORING SYSTEM Government regulate and monitor the quality of drugs through: Drugs Act, 1976 and rules under made there under. Central Licensing Board Drug Registration Board Drugs Appellate Board Provincial Quality Control Boards Inspectorate Federal and Provincial Drugs Testing Laboratories Appellate Testing Laboratory Drug Courts BY: ABDULLAH ABRO MOH PAK 36

Infrastructure Component Federal Government Main Regulatory Central Body Licensing and Registration Boards Provincial Governments Provincial Quality Control Boards Inspectors 12 238 Drug Testing Laboratories 3 4 Drug Courts 10 BY: ABDULLAH ABRO MOH PAK 37

A BRIEF ABOUT MINISTRY OF LIVESTOCK & DAIRY DEVELOPMENT • At the Federal level, Ministry of Livestock & Dairy Development formulates national policies, plans, coordinate/liaison with the Provincial / International organizations such as O. I. E Head / Regional Offices, WHO, regulates animal quarantine and inspection services, assists M/O Health in registration of veterinary drugs & biologics; collection and compilation of livestock statistics for planning national level development programs/projects for the livestock sector. BY: ABDULLAH ABRO MOH PAK 38

• Animal Quarantine Department of the Ministry of Livestock & Dairy Development, regulates the import /export of livestock and livestock products through Pakistan Animal Quarantine (Import & Export of Animals and Animal Products) Ordinance, 1979 and Rules 1980. It observes WTO, European Union and trading-partners conditionalities during export of products of animal origin. Animal Quarantine Stations are located at entry and exit points of airports and seaports, ie. Islamabad, Karachi, Lahore, Peshawar, Quetta, Multan and Sialkot. These stations have attached laboratory facilities to conduct basic microbiological diagnostic tests. The quarantine department provides Central Certification Services to importers / exporters of livestock and livestock products. BY: ABDULLAH ABRO MOH PAK 39

• Animal Quarantine Department is involved in registration of slaughterhouses, animal casing and gelatin processing units for export of these commodities. It registers these units in the light of international requirements and carries out regular inspection for pointing out their deficiencies and their rectification to produce livestock products under standard sanitary and hygiene conditions to minimize the chances of contamination in export consignments. BY: ABDULLAH ABRO MOH PAK 40

• National Veterinary Laboratory, Islamabad serves as Reference Laboratory for the diagnosis / research of livestock diseases prevalent in the country. It is also quality assurance laboratory for the export of livestock and livestock products. It provides diagnostic facilities for livestock diseases, carry out sero typing and bio typing of viruses; harmonizes disease diagnostic protocol of Provincial Veterinary Research Institute in conformity with Office International des Epizooties (OIE). It conducts tests for quality assurance for imported / exported and locally produced veterinary vaccines. It carries out drug residue testing on request in food items of animal origin. The veterinary drugs / biologicals are evaluated for their safety, potency and efficacy. It is playing a vital role in human resource development and capacity building. It arranges short term courses of national and international levels regarding new diagnostic techniques and emerging livestock diseases. It also works in close association with the provincial livestock services in analyzing, monitoring, planning and formulation of disease control and eradication programs. BY: ABDULLAH ABRO MOH PAK 41

• The National Reference Laboratory for Poultry Diseases (NRLPD) located at the NARC Islamabad provides diagnostic facilities for poultry diseases, carries out research on local virus isolates and harmonizes disease diagnostic protocol of VRIs / PRIs in conformity with OIE. It is also playing a vital role in human resource development and capacity building. It arranges short term courses of national and international levels regarding new diagnostic techniques and emerging poultry diseases like Avian Influenza. The NRLPD also works in close association with the provincial livestock services in analyzing, monitoring, planning and formulation of disease control and eradication programs. BY: ABDULLAH ABRO MOH PAK 42

• Provincial livestock departments are the executing agencies and focal points for all the livestock activities in the provinces. The provincial livestock departments are divided into Extension Directorate, Breed Improvement Directorate, Animal Health Directorate, Livestock Farm Directorate, Planning Department and Research Directorates. These directorates function in their defined spheres through a net work of field offices at gross root level. The Federal and Provincial veterinary authorities interact with each other periodically. BY: ABDULLAH ABRO MOH PAK 43

• Provincial livestock departments have their own set ups generally comprising of Senior level Administrative Staff (DGs/Directors/Project Directors), District level Administrative Staff (DLOs/DDs/Ads), Field Veterinary Officers (V. O) and para-veterinary staff like Livestock Assistants/SA, AI Technicians and Veterinary Compounders etc. Currently, there are 5 DGs and 31 Directors/Project Directors working in provincial Livestock Departments in the country. The district level administrative staff consists of 89 DLOs/DDs and 198 SVOs/ADs. Nearly 2300 field Veterinary Officers are working in different provinces/areas of the country. These field veterinary officers are supported by more than 5400 para-veterinary staff. BY: ABDULLAH ABRO MOH PAK 44

THANKS BY: ABDULLAH ABRO MOH PAK 45