Worksheet Chapter 12 Homework Key 1 Determine whether


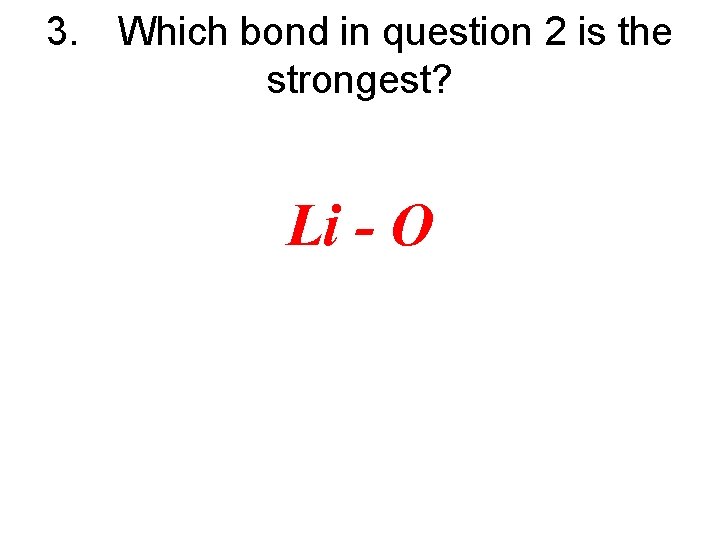








- Slides: 20

Worksheet: Chapter 12 Homework Key

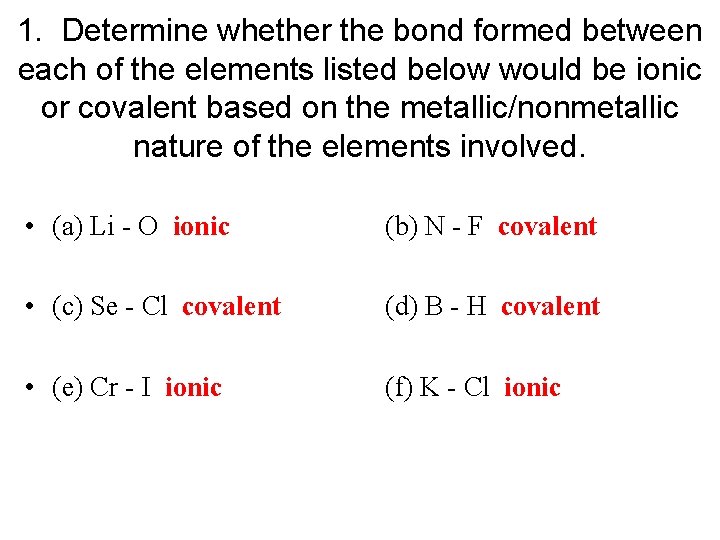
1. Determine whether the bond formed between each of the elements listed below would be ionic or covalent based on the metallic/nonmetallic nature of the elements involved.

1. Determine whether the bond formed between each of the elements listed below would be ionic or covalent based on the metallic/nonmetallic nature of the elements involved. • (a) Li - O ionic (b) N - F covalent • (c) Se - Cl covalent (d) B - H covalent • (e) Cr - I ionic (f) K - Cl ionic

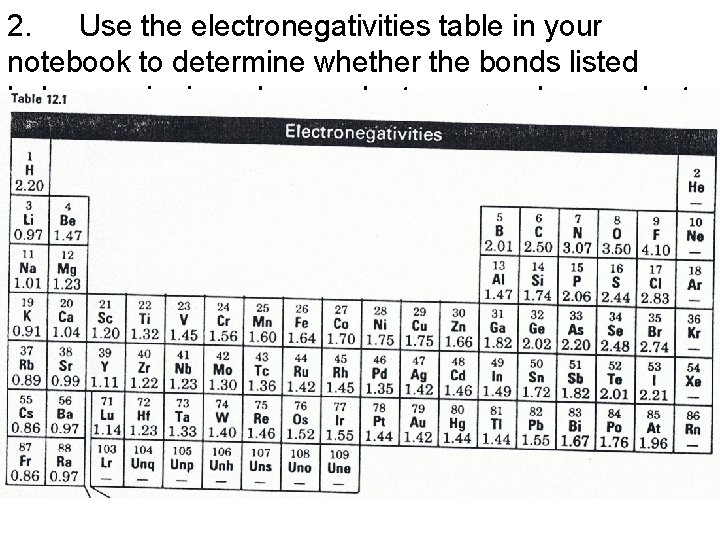
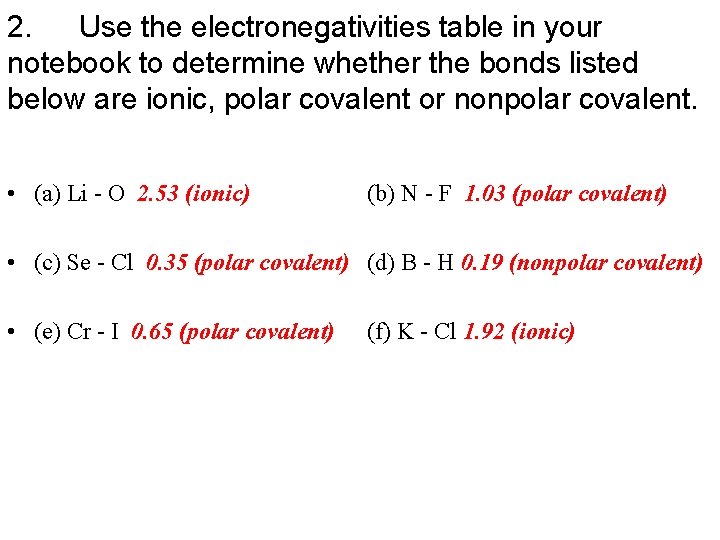
2. Use the electronegativities table in your notebook to determine whether the bonds listed below are ionic, polar covalent or nonpolar covalent. .

2. Use the electronegativities table in your notebook to determine whether the bonds listed below are ionic, polar covalent or nonpolar covalent. • (a) Li - O 2. 53 (ionic) (b) N - F 1. 03 (polar covalent) • (c) Se - Cl 0. 35 (polar covalent) (d) B - H 0. 19 (nonpolar covalent) • (e) Cr - I 0. 65 (polar covalent) (f) K - Cl 1. 92 (ionic)
3. Which bond in question 2 is the strongest?
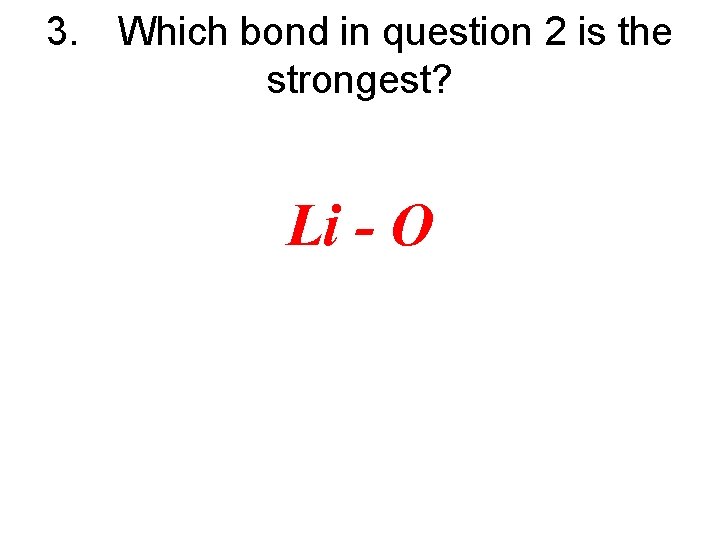
3. Which bond in question 2 is the strongest? Li - O

4. List three properties of metals.

4. List three properties of metals. • • • high melting points (solids) hard conductors shiny malleable lose electrons

5. Metallic bonds have “delocalized electrons”. What are delocalized electrons?

5. Metallic bonds have “delocalized electrons”. What are delocalized electrons? • Delocalized electrons are electrons which are free to drift from one atom to another within a metal.

6. Why do covalent bonds form between elements with similar electronegativities?

6. Why do covalent bonds form between elements with similar electronegativities? • Elements with similar electronegativities have to share electrons because neither atom has enough pull on the electrons (electronegativity) to capture the electrons from the other atom.

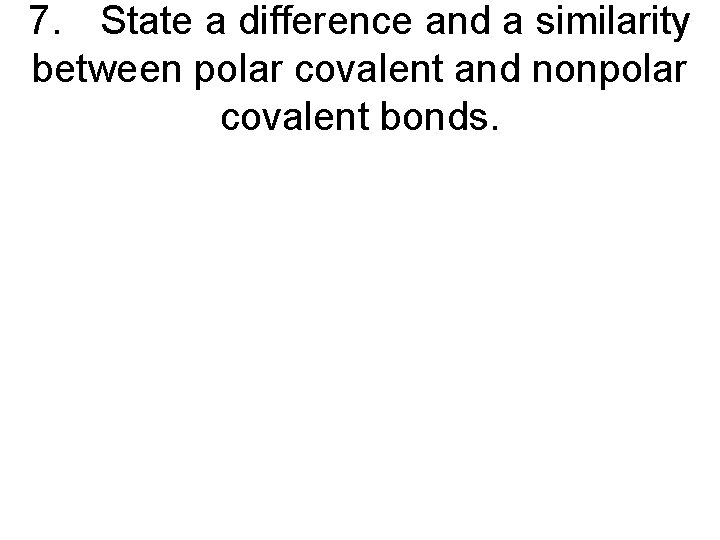
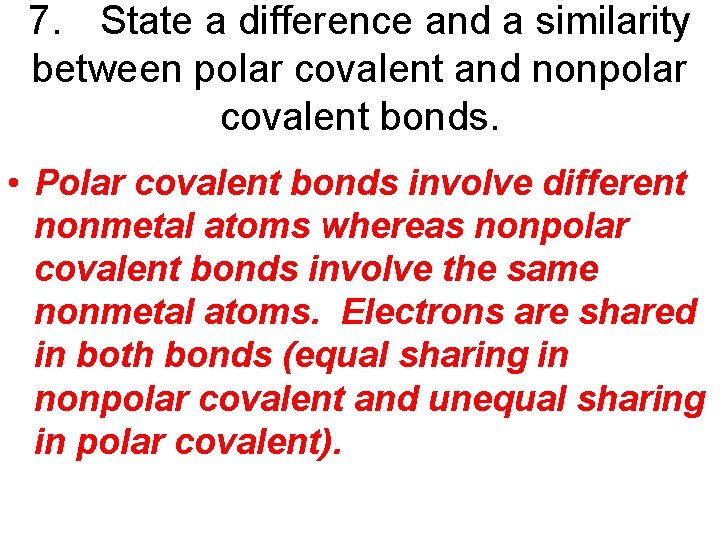
7. State a difference and a similarity between polar covalent and nonpolar covalent bonds.

7. State a difference and a similarity between polar covalent and nonpolar covalent bonds. • Polar covalent bonds involve different nonmetal atoms whereas nonpolar covalent bonds involve the same nonmetal atoms. Electrons are shared in both bonds (equal sharing in nonpolar covalent and unequal sharing in polar covalent).

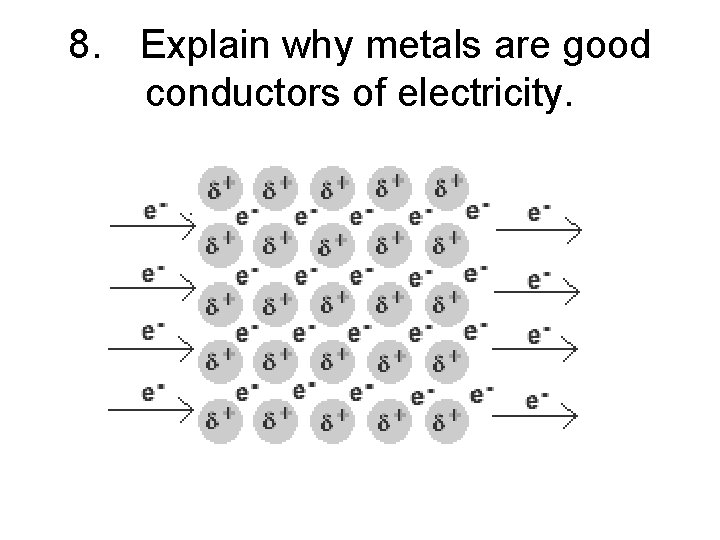
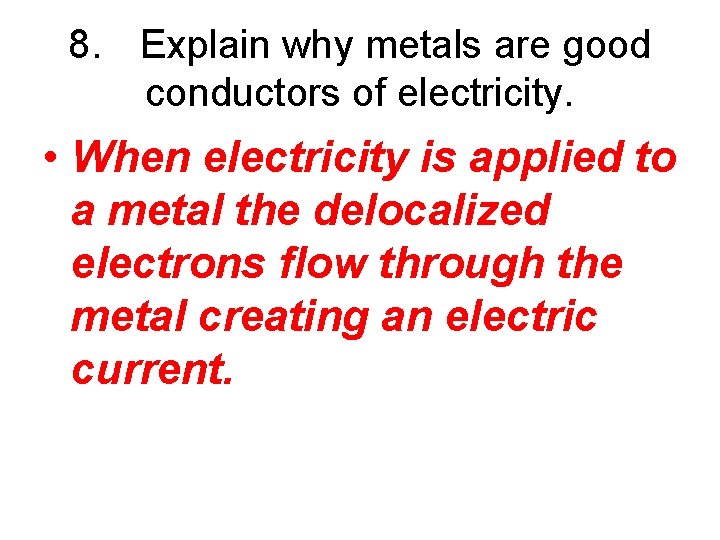
8. Explain why metals are good conductors of electricity.

8. Explain why metals are good conductors of electricity.

8. Explain why metals are good conductors of electricity. • When electricity is applied to a metal the delocalized electrons flow through the metal creating an electric current.

9. Why are metals malleable whereas ionic compounds are brittle?

9. Why are metals malleable whereas ionic compounds are brittle? • When a metal is bent and the atoms slide past one another the delocalized electrons rearrange themselves to reform bonds when old bonds are broken. In an ionic compound the electrons are localized creating fixed positive and negative ions and when these ions slide past one another bonds do not have the ability to reform and the substance shatters when ions of like charges get next to each other.