Worked Example 13 1 Naming Organic Compounds Alkenes




- Slides: 16

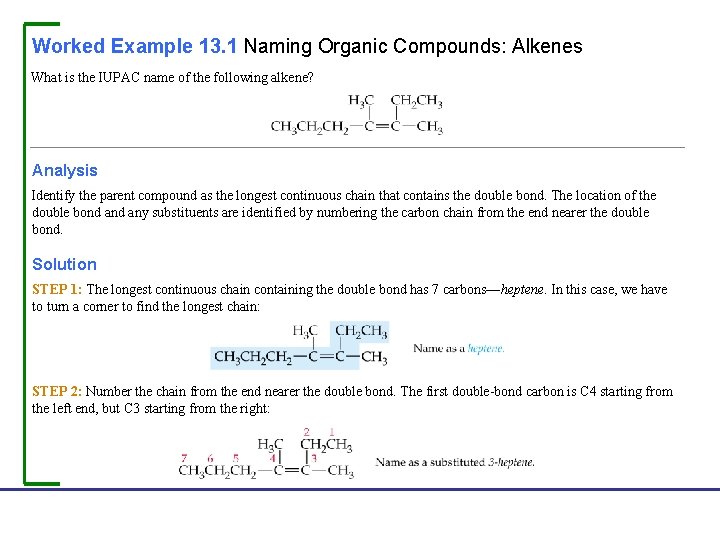
Worked Example 13. 1 Naming Organic Compounds: Alkenes What is the IUPAC name of the following alkene? Analysis Identify the parent compound as the longest continuous chain that contains the double bond. The location of the double bond any substituents are identified by numbering the carbon chain from the end nearer the double bond. Solution STEP 1: The longest continuous chain containing the double bond has 7 carbons—heptene. In this case, we have to turn a corner to find the longest chain: STEP 2: Number the chain from the end nearer the double bond. The first double-bond carbon is C 4 starting from the left end, but C 3 starting from the right: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

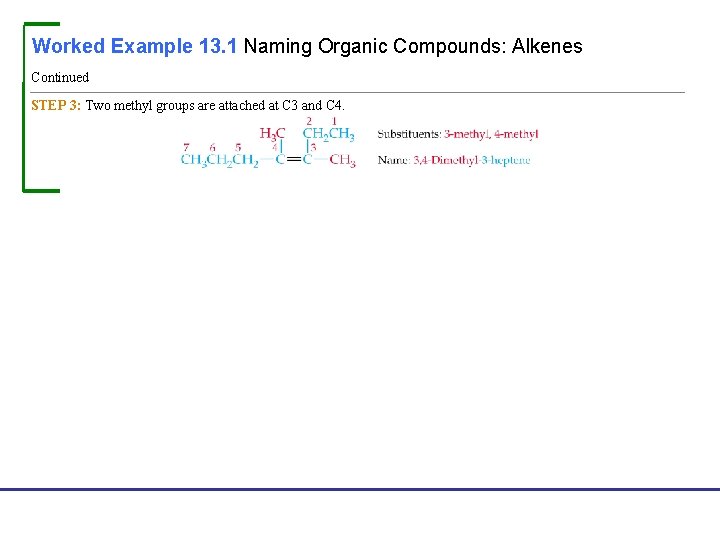
Worked Example 13. 1 Naming Organic Compounds: Alkenes Continued STEP 3: Two methyl groups are attached at C 3 and C 4. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

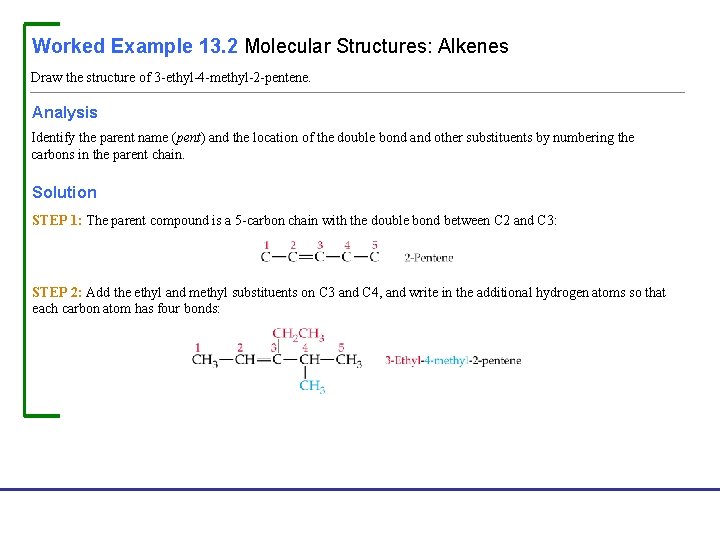
Worked Example 13. 2 Molecular Structures: Alkenes Draw the structure of 3 -ethyl-4 -methyl-2 -pentene. Analysis Identify the parent name (pent) and the location of the double bond and other substituents by numbering the carbons in the parent chain. Solution STEP 1: The parent compound is a 5 -carbon chain with the double bond between C 2 and C 3: STEP 2: Add the ethyl and methyl substituents on C 3 and C 4, and write in the additional hydrogen atoms so that each carbon atom has four bonds: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

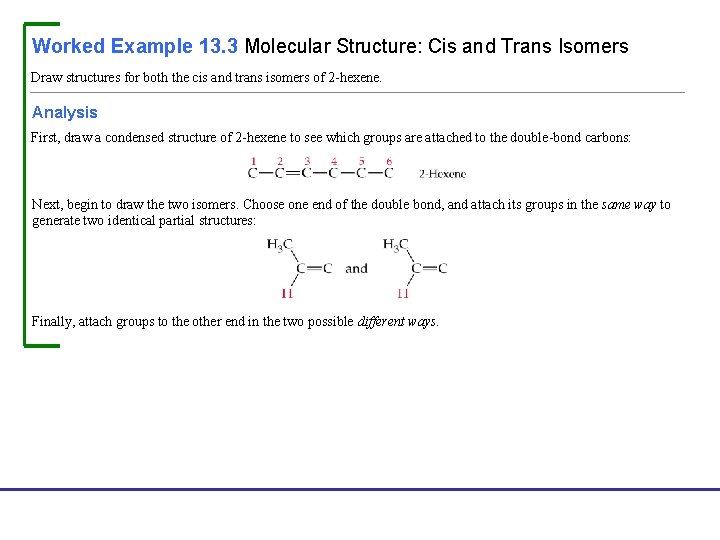
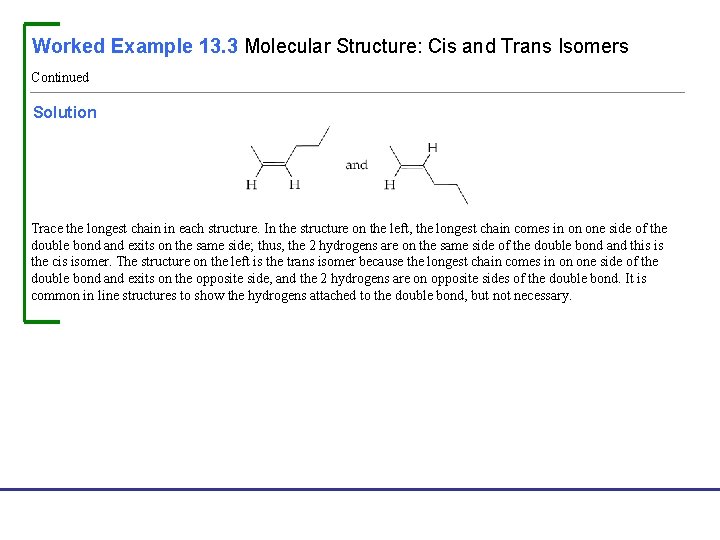
Worked Example 13. 3 Molecular Structure: Cis and Trans Isomers Draw structures for both the cis and trans isomers of 2 -hexene. Analysis First, draw a condensed structure of 2 -hexene to see which groups are attached to the double-bond carbons: Next, begin to draw the two isomers. Choose one end of the double bond, and attach its groups in the same way to generate two identical partial structures: Finally, attach groups to the other end in the two possible different ways. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 3 Molecular Structure: Cis and Trans Isomers Continued Solution Trace the longest chain in each structure. In the structure on the left, the longest chain comes in on one side of the double bond and exits on the same side; thus, the 2 hydrogens are on the same side of the double bond and this is the cis isomer. The structure on the left is the trans isomer because the longest chain comes in on one side of the double bond and exits on the opposite side, and the 2 hydrogens are on opposite sides of the double bond. It is common in line structures to show the hydrogens attached to the double bond, but not necessary. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 4 Identifying Reactions of Alkenes Classify the following alkene reactions as addition, elimination, or substitution reactions: Analysis Determine whether atoms have been added to the starting compound (addition), removed from the starting compound (elimination), or switched with another reactant (substitution). Solution (a) Two H atoms have been added in place of the double bond, so this is an addition reaction. (b) A water molecule (H 2 O) has been formed by removing an H atom and an —OH group from adjacent C atoms, forming a double bond in the process, so this is an elimination reaction. (c) The reactants (CH 3 CH 2 Cl and KOH) have traded the —OH and the —Cl substituent groups, so this is a substitution reaction. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

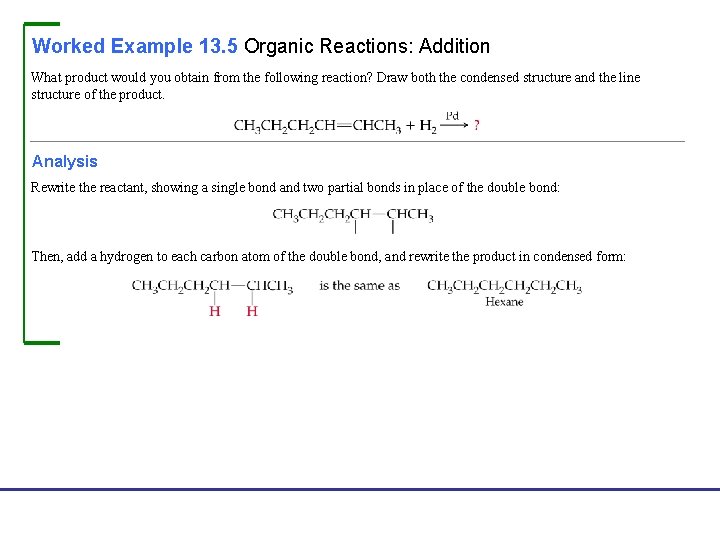
Worked Example 13. 5 Organic Reactions: Addition What product would you obtain from the following reaction? Draw both the condensed structure and the line structure of the product. Analysis Rewrite the reactant, showing a single bond and two partial bonds in place of the double bond: Then, add a hydrogen to each carbon atom of the double bond, and rewrite the product in condensed form: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 5 Organic Reactions: Addition Continued Solution The reaction is In line structure format, this reaction would look as follows: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

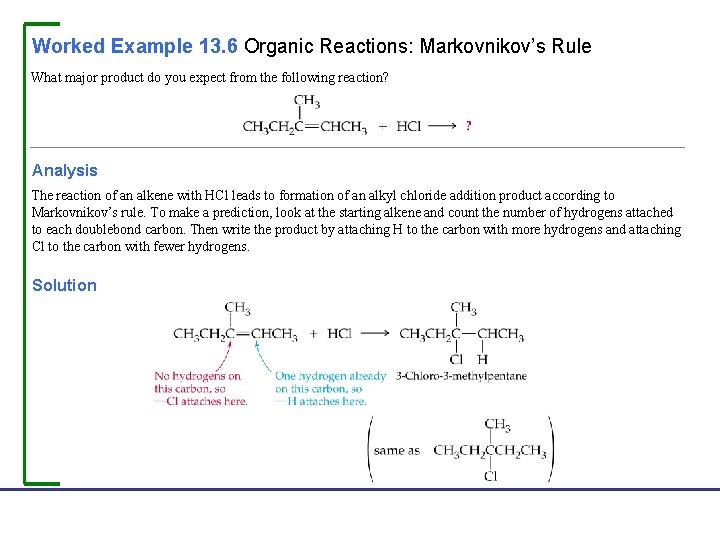
Worked Example 13. 6 Organic Reactions: Markovnikov’s Rule What major product do you expect from the following reaction? Analysis The reaction of an alkene with HCl leads to formation of an alkyl chloride addition product according to Markovnikov’s rule. To make a prediction, look at the starting alkene and count the number of hydrogens attached to each doublebond carbon. Then write the product by attaching H to the carbon with more hydrogens and attaching Cl to the carbon with fewer hydrogens. Solution Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

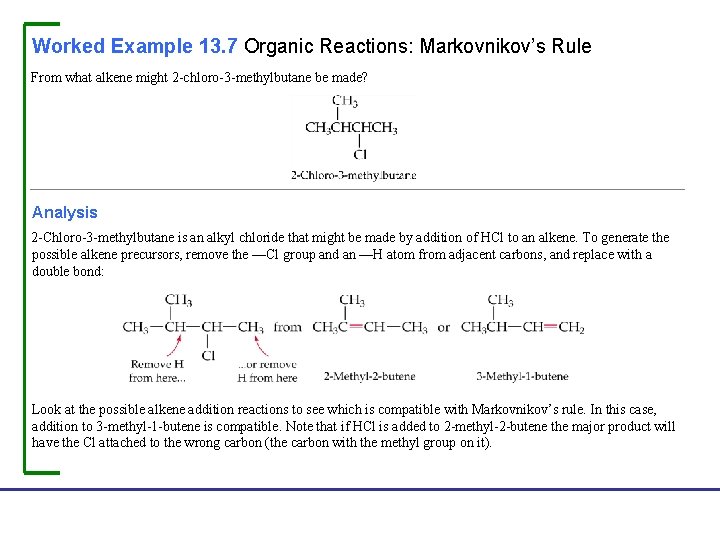
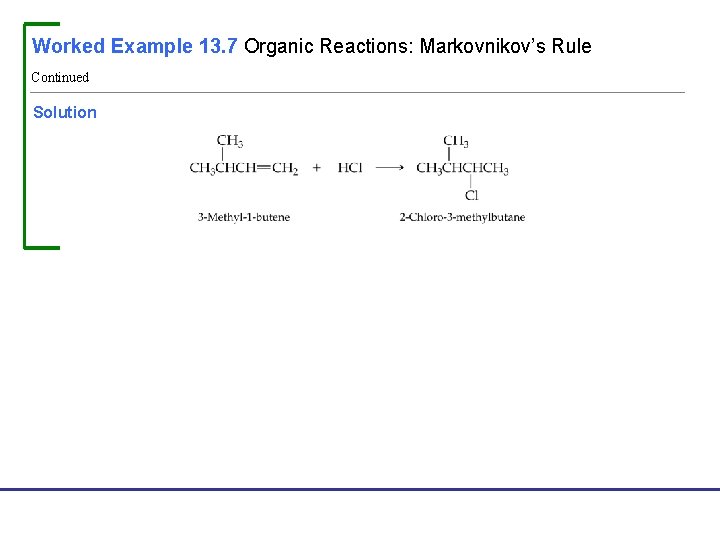
Worked Example 13. 7 Organic Reactions: Markovnikov’s Rule From what alkene might 2 -chloro-3 -methylbutane be made? Analysis 2 -Chloro-3 -methylbutane is an alkyl chloride that might be made by addition of HCl to an alkene. To generate the possible alkene precursors, remove the —Cl group and an —H atom from adjacent carbons, and replace with a double bond: Look at the possible alkene addition reactions to see which is compatible with Markovnikov’s rule. In this case, addition to 3 -methyl-1 -butene is compatible. Note that if HCl is added to 2 -methyl-2 -butene the major product will have the Cl attached to the wrong carbon (the carbon with the methyl group on it). Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 7 Organic Reactions: Markovnikov’s Rule Continued Solution Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

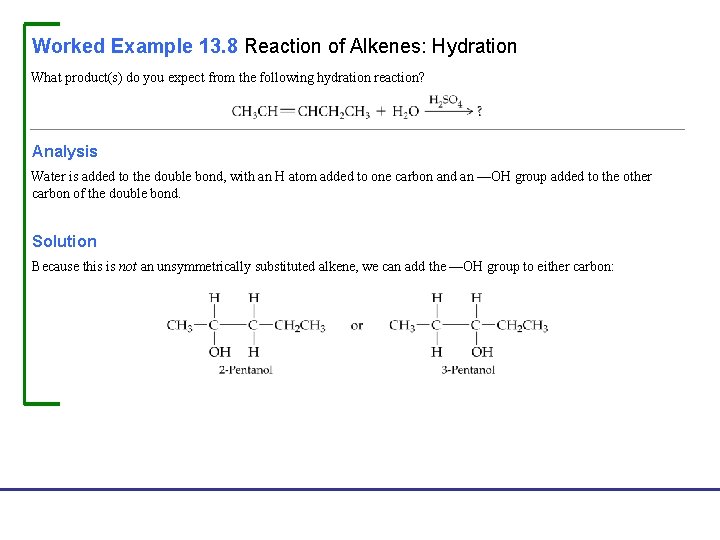
Worked Example 13. 8 Reaction of Alkenes: Hydration What product(s) do you expect from the following hydration reaction? Analysis Water is added to the double bond, with an H atom added to one carbon and an —OH group added to the other carbon of the double bond. Solution Because this is not an unsymmetrically substituted alkene, we can add the —OH group to either carbon: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 9 Reactions of Alkenes: Polymerization Write the structure of a segment of polystyrene, used in foams and molded plastics. The monomer is Analysis The polymerization reaction resembles the addition of two monomer units to either end of the double bond. Solution Draw three molecules of styrene with the double bonds aligned next to each other; then add the monomer units together with single bonds, eliminating the double bonds in the process: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

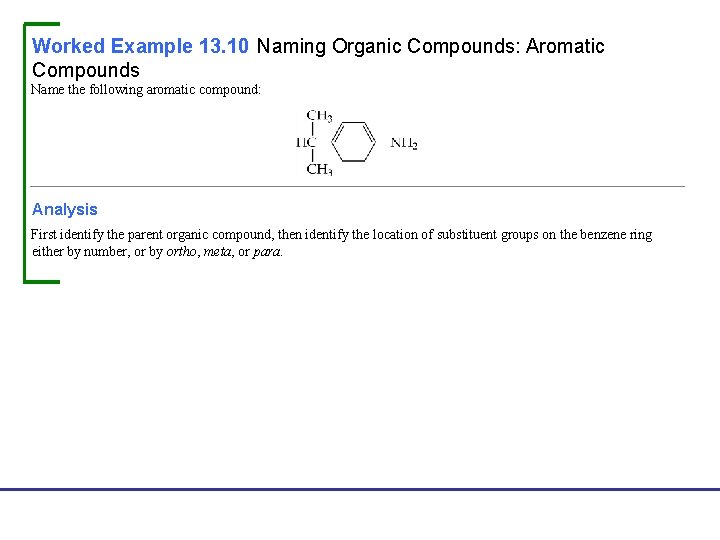
Worked Example 13. 10 Naming Organic Compounds: Aromatic Compounds Name the following aromatic compound: Analysis First identify the parent organic compound, then identify the location of substituent groups on the benzene ring either by number, or by ortho, meta, or para. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

Worked Example 13. 10 Naming Organic Compounds: Aromatic Compounds Continued Solution The parent compound is a benzene ring with an amine group (aminobenzene, which is commonly known as aniline). The substituent group is attached at the C 4, or para, position relative to the amino group. The propyl group is attached to the benzene ring by the middle carbon, so it is isopropyl. Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.

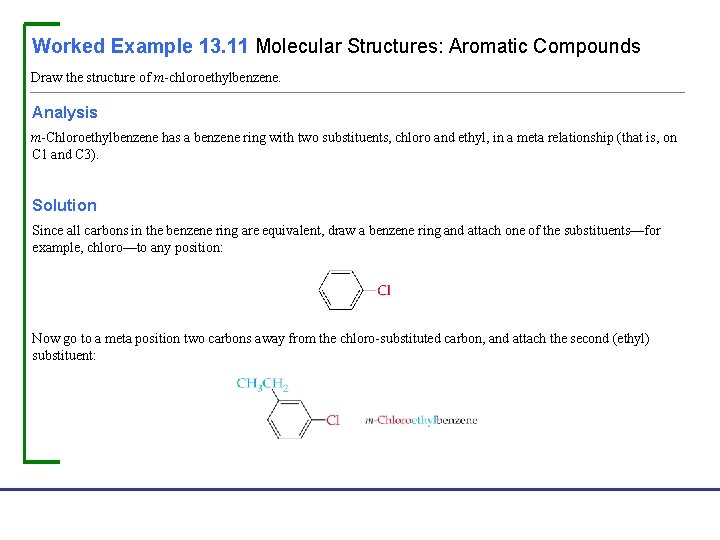
Worked Example 13. 11 Molecular Structures: Aromatic Compounds Draw the structure of m-chloroethylbenzene. Analysis m-Chloroethylbenzene has a benzene ring with two substituents, chloro and ethyl, in a meta relationship (that is, on C 1 and C 3). Solution Since all carbons in the benzene ring are equivalent, draw a benzene ring and attach one of the substituents—for example, chloro—to any position: Now go to a meta position two carbons away from the chloro-substituted carbon, and attach the second (ethyl) substituent: Fundamentals of General, Organic, and Biological Chemistry, 7 e John Mc. Murry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson © 2013 Pearson Education, Inc.