Work and energy a give examples of energy

























- Slides: 27

Work and energy (a) give examples of energy in different forms, its conversion and conservation, and apply the principle of energy conservation to simple Examples (e) recall and apply the formula Ek = ½ mv 2 (i) recall and use the formula Ep = mgh for potential energy changes near the Earth’s surface

Introduction • Think of force as a mechanism by which energy is transferred from one body to another. • This only occurs when the object moves in the direction of the force. • Consider the forces on vehicles when accelerating/decelerating. • Consider the effect of the distance over which the force acts rather than the time of action of the force.

GREEN SICK • • • Gravitational Radiative (e-m waves) Electrical Elastic Nuclear Sound Internal (Thermal) Chemical Kinetic That’s it Really no other types exist Except maybe dark energy but that is another story

Energy transfers • Write the 9 types of energy in a circle on a double page in your notebooks. • Draw a straight line from one form of energy to any other and add an arrow • Write on the arrow a device or phenomenon that causes that change.

Kinetic Energy • This is the energy that a body possesses by virtue of its motion. • If the mass of a body is m and its velocity is v then its kinetic energy is ……. . • Ek = ½ m 2 v

Gravitational Potential Energy • This is the energy that a body possesses by virtue of its position in the gravitational field • If the mass of a body is m and its height above a fixed position is h then its change in gravitational potential energy…………. • Ep = mg h • where g = the acceleration due to gravity

The Principle of Conservation of Energy • Energy can be transformed from one form to another, but it cannot be created nor destroyed. • i. e. the total energy of a system is constant • Energy is measured in joules and it is a scalar quantity

Examples • Falling objects and roller coaster rides are situations where Ep + Ek = constant • If we ignore the effects of air resistance and friction. • Inclined planes and falling objects can often be solved more simply using this principle rather than the kinematics equations

Collisions and explosions • In all collisions and explosions momentum is conserved. (see momentum) • Generally there is a loss of kinetic energy, usually to internal energy (heat) and to a lesser extent to sound • In an inelastic collision there is a loss of kinetic energy (momentum is still conserved) • In an elastic collision the kinetic energy is conserved (as well as momentum)

KE in collisions • In elastic collisions kinetic energy is conserved. • Total KE before collision = Total KE after collision • In inelastic collisions kinetic energy is ‘lost’. • Total KE before collision > Total KE after collision

Work • (b) show an understanding of the concept of work in terms of the product of a force and displacement in the direction of the force • (c) calculate the work done in a number of situations including the work done by a gas that is expanding against a constant external pressure: W = p ΔV • (h) derive, from the defining equation W = Fs, the formula Ep = mgh for potential energy changes near the Earth’s surface

Work • We say that work is done by a force when the object concerned moves in the direction of the force, and the force thereby transfers energy from one object to another. • Consider the simplest possible case of work done. A force ‘F’ is acting on an object. The object has a displacement ‘S’ in the direction of the force. Then the work done is the product of force and displacement.

Work • What will happen in the case when the applied force is not in the direction of displacement but rather at an angle to it. • Find the component of force in the direction of displacement. • This component will be doing work. • Component of force in the direction of displacement is F Cos θ. • W = (F Cos θ) S = F. s • Work done is a scalar quantity.

An illustrative example • • • What sort of energy is gained by raising a mass ? GPE What is the force acting against the movement? Weight (mg) We say that the lifting force is doing work against gravity. If the object is raised at a steady speed, it is in equilibrium and the lifting force will just balance weight. This of course ignores any air resistance What is g? Gravitational field strength (Force per unit mass

• What two factors will determine how much energy you lose (and hence the energy transferred to the object)? • The size of the force and the distance h moved in the direction of the force. • Can you state the equation which gives the energy gained? • mgh • Hence how much energy is required to lift object height h? • mgh • This suggests that energy transferred = force distance moved in direction of force. • This is known as the work done by the force. • The equation GPE = mgh • mgh is simply a particular case of work done.

Another example • What happens to the energy if the mass is pushed along a surface? • Work is done against a frictional force. • The energy transferred ends up as heat.

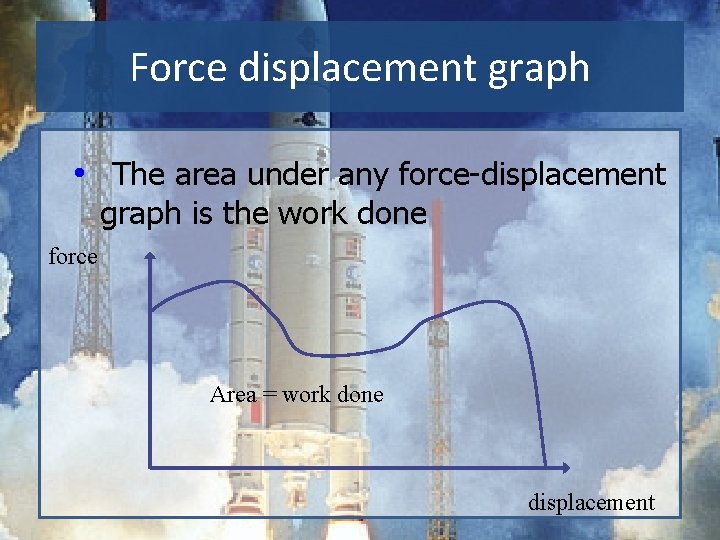
Force displacement graph • The area under any force-displacement graph is the work done force Area = work done displacement

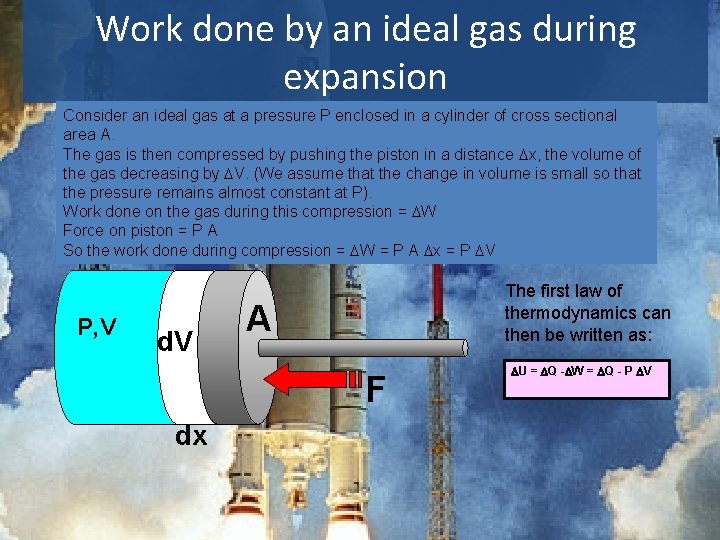
Work done by an ideal gas during expansion Consider an ideal gas at a pressure P enclosed in a cylinder of cross sectional area A. The gas is then compressed by pushing the piston in a distance Dx, the volume of the gas decreasing by DV. (We assume that the change in volume is small so that the pressure remains almost constant at P). Work done on the gas during this compression = DW Force on piston = P A So the work done during compression = DW = P A Dx = P DV P, V d. V The first law of thermodynamics can then be written as: A F dx DU = DQ -DW = DQ - P DV

To do • P 67 q 2 and 5 • P 108 q 36, 38, 39

Kinetic Energy • (d) derive, from the equations of motion, the formula Ek = ½ mv 2

Derivation • • • See p 63 in P 4 U Use v 2=u 2+2 as, F=ma and Work =Fs v 2=u 2+2 as Rearrange for a a = (v 2 -u 2)/2 s Now use F=ma = m (v 2 -u 2)/2 s Now use Work = Fs = sm (v 2 -u 2)/2 s Cancel the s Work = m (v 2 -u 2)/2 Work = Energy transferred = Ek = m (v 2 -u 2)/2 For u= 0 Ek = m v 2/2

Energy and efficiency • (f) distinguish between gravitational potential energy, electric potential energy and elastic potential energy • (g) show an understanding and use the relationship between force and potential energy in a uniform field to solve problems • (j) show an understanding of the concept of internal energy • (k) show an appreciation for the implications of energy losses in practical devices and use the concept of efficiency to solve problems

Potential Energy • Potential energy is “stored” by a body • Gravitational Potential Energy– due to position of body in a gravitational field (mgh) • Electrical potential Energy – due to position of a charged particle in an electric field (q. Ed) • Elastic Potential energy – due to the work done against a restoring force (tension or compression) (Average force x displacement) • Note all 3 equations are effectively F=ma

Force and potential energy • We have already seen examples where work done = Potential energy stored for gravitational fields. • The same type of calculation can be used for potential energy in Uniform electric fields (between parrallel plates) • And more obviously for elastic potential energy problems.

Internal energy (U) • Internal energy (often incorrectly called Heat) is the total energy of an object due to the Kinetic and potential energies of it, s particles. • For ideal gasses there is no Potential element to internal energy • Why is this? • For all other materials U is the sum of KE and PE for all of the particles.

Some helpful examples • Hotness means temperature not energy • E. g. A spark at 1000 K contains less energy than a cup of tea at 330 K • Particles in different phases of matter have differrent amounts of potential energy so different internal energies. • E. g. A steam burn is worse than a water burn 1 kg of “steam” at 100 C has greater internal energy than 1 kg of water at 100 C. (due to PE of particles)

Efficiency • • Useful energy out / total energy in Always less than 1 Often quoted as percentage Can be represented on a Sankey diagram see p 65