WM 4 Instrumental analysis The 3 key instrumental







- Slides: 22

WM 4 Instrumental analysis

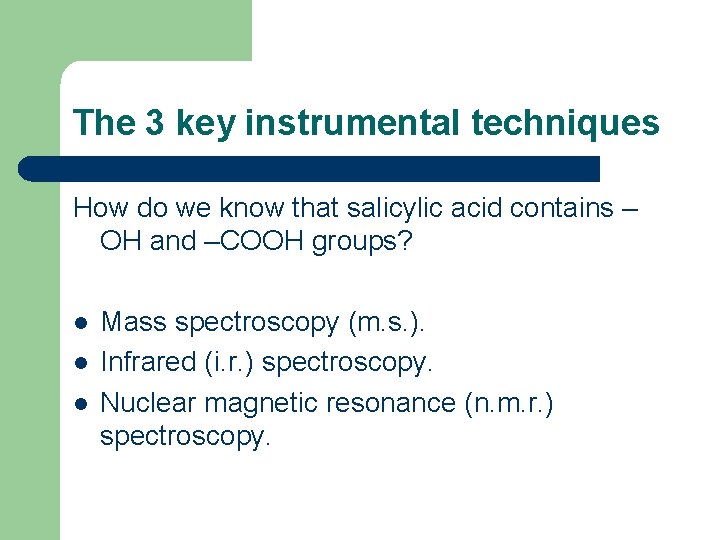
The 3 key instrumental techniques How do we know that salicylic acid contains – OH and –COOH groups? l l l Mass spectroscopy (m. s. ). Infrared (i. r. ) spectroscopy. Nuclear magnetic resonance (n. m. r. ) spectroscopy.

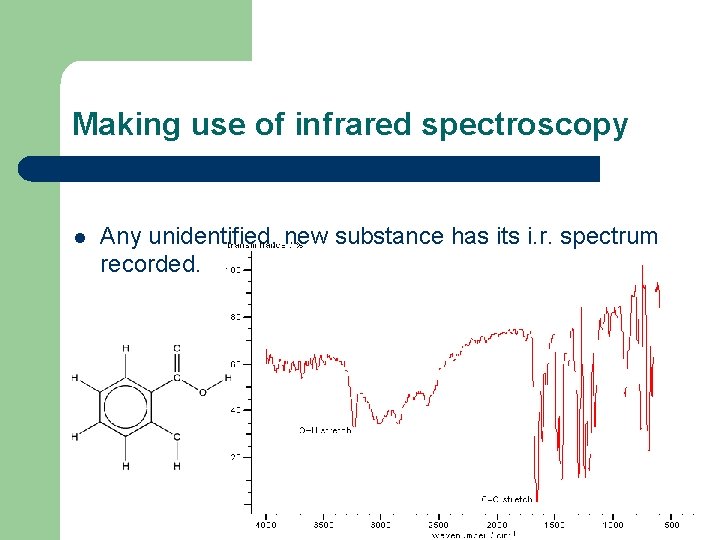
Making use of infrared spectroscopy l Any unidentified, new substance has its i. r. spectrum recorded.

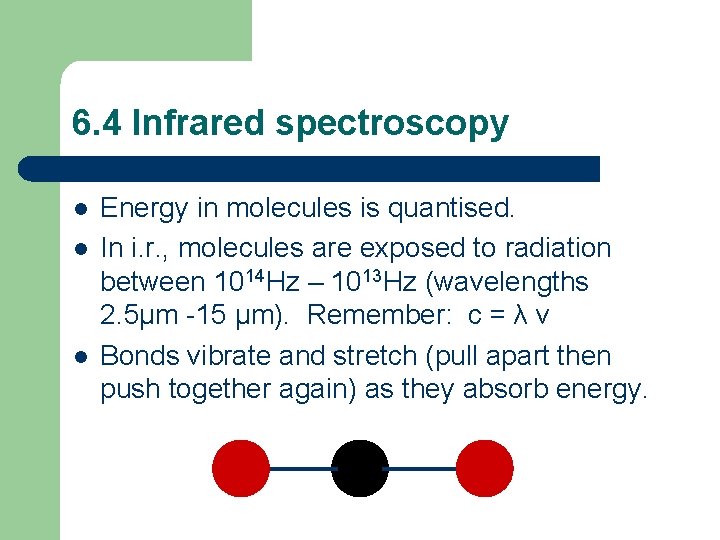
6. 4 Infrared spectroscopy l l l Energy in molecules is quantised. In i. r. , molecules are exposed to radiation between 1014 Hz – 1013 Hz (wavelengths 2. 5µm -15 µm). Remember: c = λ v Bonds vibrate and stretch (pull apart then push together again) as they absorb energy.

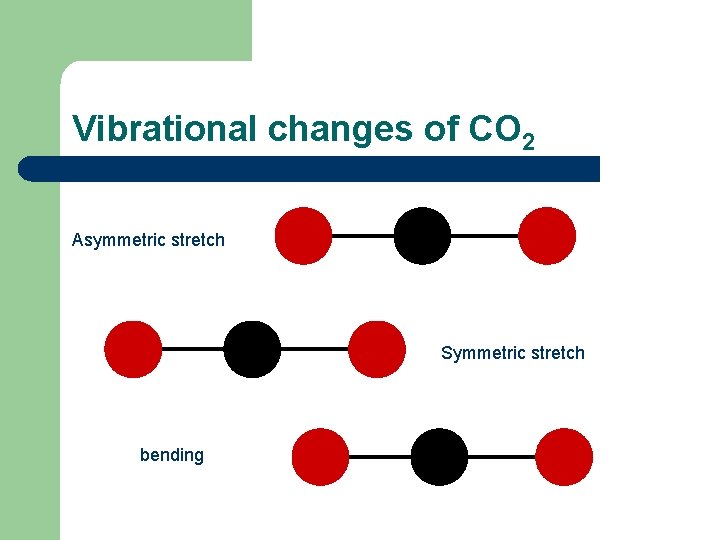
Vibrational changes of CO 2 Asymmetric stretch Symmetric stretch bending

Infrared spectra: signals = stretches l l l An IR spectrum hangs down from a baseline (100% transmittance = no absorbtion). The signals (look like ‘icicles’) on an IR spectra correspond to bonds absorbing a packet of energy and vibrating more. The –OH and –CO bonds in salicylic acid absorb energy at specific wavelengths (λ)/µm and so wavenumbers (1/ λ)/ cm-1.

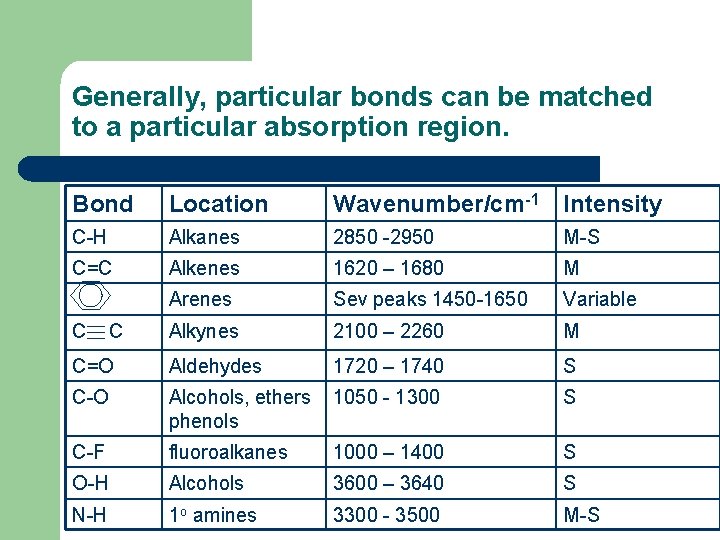
Generally, particular bonds can be matched to a particular absorption region. Bond Location Wavenumber/cm-1 Intensity C-H Alkanes 2850 -2950 M-S C=C Alkenes 1620 – 1680 M Arenes Sev peaks 1450 -1650 Variable Alkynes 2100 – 2260 M C=O Aldehydes 1720 – 1740 S C-O Alcohols, ethers phenols 1050 - 1300 S C-F fluoroalkanes 1000 – 1400 S O-H Alcohols 3600 – 3640 S N-H 1 o amines 3300 - 3500 M-S C C

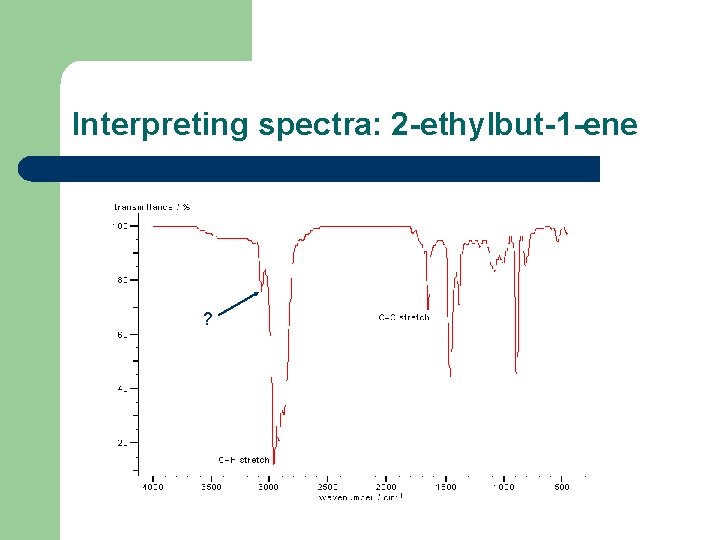
Interpreting spectra: 2 -ethylbut-1 -ene ?

Interpreting spectra: propanone

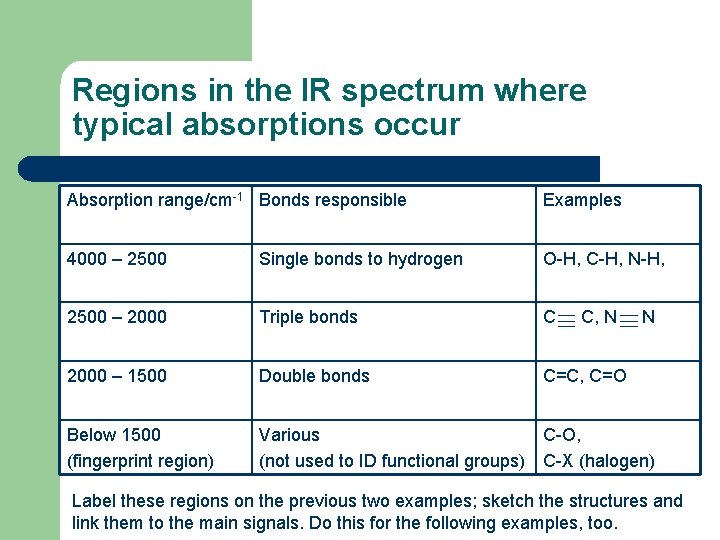
Regions in the IR spectrum where typical absorptions occur Absorption range/cm-1 Bonds responsible Examples 4000 – 2500 Single bonds to hydrogen O-H, C-H, N-H, 2500 – 2000 Triple bonds C 2000 – 1500 Double bonds C=C, C=O Below 1500 (fingerprint region) Various C-O, (not used to ID functional groups) C-X (halogen) C, N N Label these regions on the previous two examples; sketch the structures and link them to the main signals. Do this for the following examples, too.

Examples of infrared spectra Butane l l Strong absorption at 2970 cm-1 characteristic of C-H stretching in aliphatic compounds. No indication of any functional groups.

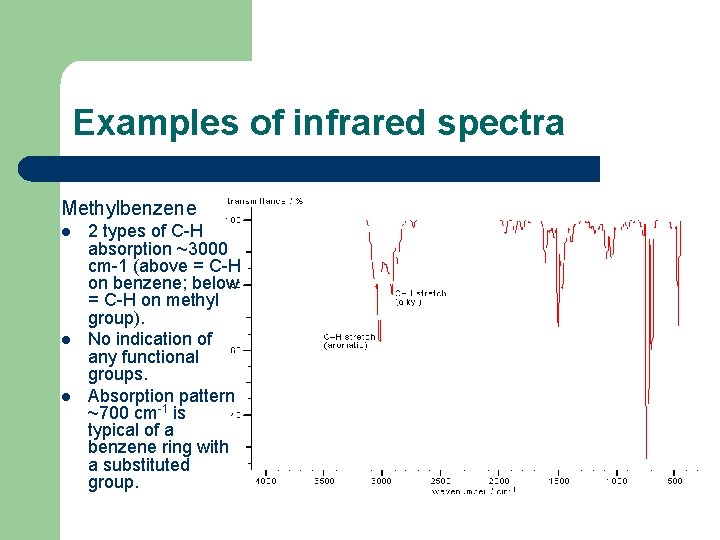
Examples of infrared spectra Methylbenzene l l l 2 types of C-H absorption ~3000 cm-1 (above = C-H on benzene; below = C-H on methyl group). No indication of any functional groups. Absorption pattern ~700 cm-1 is typical of a benzene ring with a substituted group.

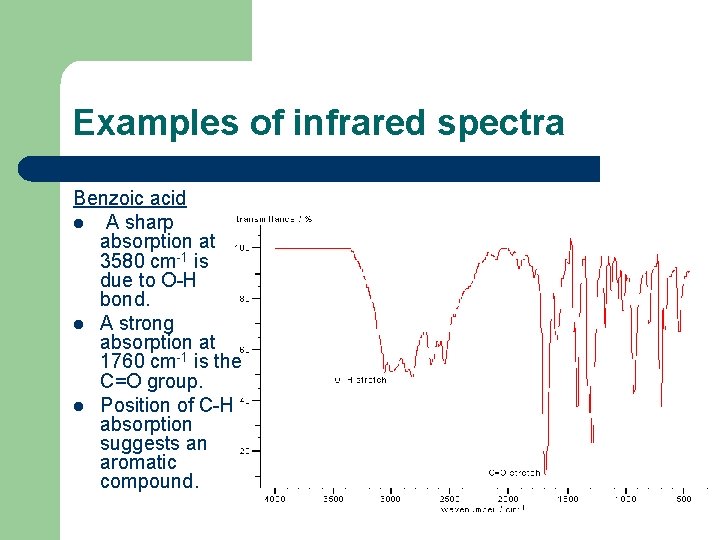
Examples of infrared spectra Benzoic acid l A sharp absorption at 3580 cm-1 is due to O-H bond. l A strong absorption at 1760 cm-1 is the C=O group. l Position of C-H absorption suggests an aromatic compound.

Summary of IR spectroscopy l l l An IR spectrum measures the extent to which electromagnetic radiation is transmitted through a sample of substance. Frequency ranges absorbed give clues about functional groups which are present. IR spectrum of salicylic acid gives evidence of C=O and –OH groups.

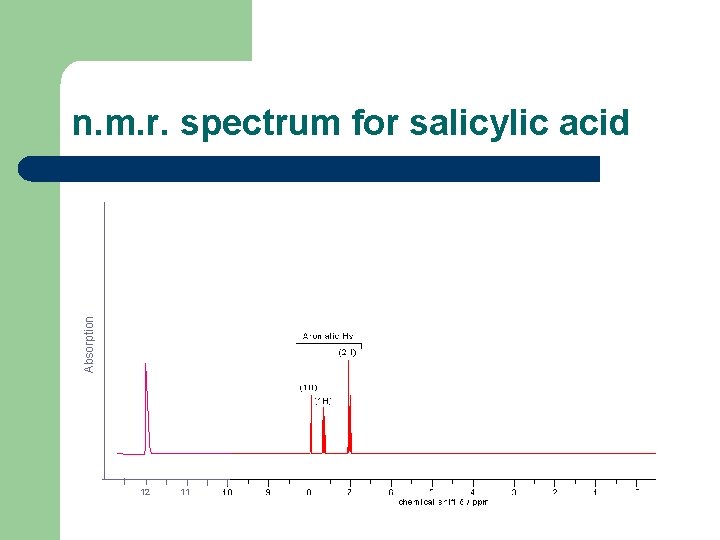
Evidence from nuclear magnetic resonance (n. m. r. ) spectroscopy. l l This technique helps to determine structure, as it investigates the different environments in which (hydrogen) nuclei are situated. The n. m. r. spectrum for salicylic acid shows signals for the different environments of the 6 protons: One proton in a –COOH environment. One proton in a phenolic –OH environment. Four protons attached to a benzene ring.

Absorption n. m. r. spectrum for salicylic acid 12 11

The evidence so far…. l l l A combination of i. r. and n. m. r. spectroscopy shows that salicylic acid has an –OH group and –COOH group both attached to a benzene ring; we can now rename it HYDROXYBENZOIC ACID. However, it could be one of 3 possible isomers: 2 -hydroxybenzoic acid, 3 hydroxybenzoic acid and 4 -hydroxybenzoic acid. Mass spectroscopy can determine which isomer we have.

The mass spectrum of salicylic acid l Signals correspond to positively charged ions formed from the parent compound, and fragment ions. The fragmentation pattern is characteristic of a particular compound…the fragment at 120 can only come from 2 hydroxybenzoic acid…can you see why? mass Parent or molecular ion

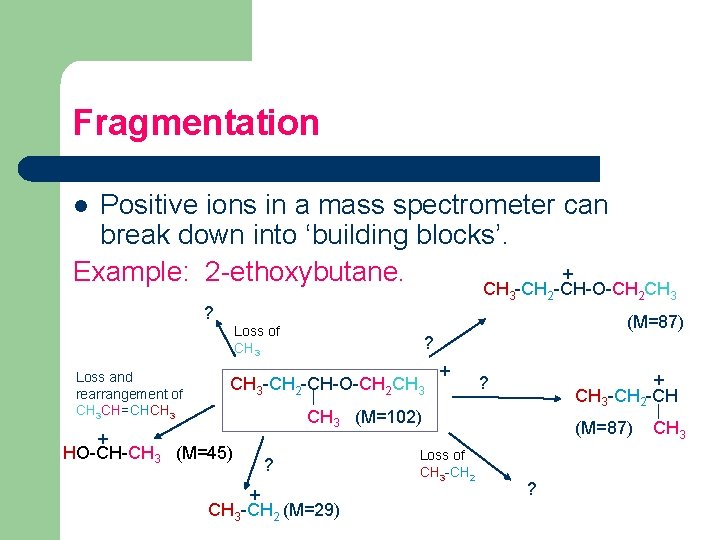
Fragmentation Positive ions in a mass spectrometer can break down into ‘building blocks’. + Example: 2 -ethoxybutane. l CH 3 -CH 2 -CH-O-CH 2 CH 3 ? (M=87) Loss of CH 3 Loss and rearrangement of CH 3 CH=CHCH 3 ? CH 3 -CH 2 -CH-O-CH 2 CH 3 + HO-CH-CH 3 (M=45) + + CH 3 -CH 2 -CH ? CH 3 (M=102) ? + CH 3 -CH 2 (M=29) Loss of CH 3 -CH 2 (M=87) ? CH 3

Positively charged fragments form. Mass difference suggests functional groups. For each fragmentation, one product has a positive charge: M+ A+ + B A + B+ The most stable ion usually forms. Mass Difference Group that is suggested 15 CH 3 17 OH 28 C=O or C 2 H 4 29 C 2 H 5 43 COCH 3 45 COOH 77 C 6 H 5

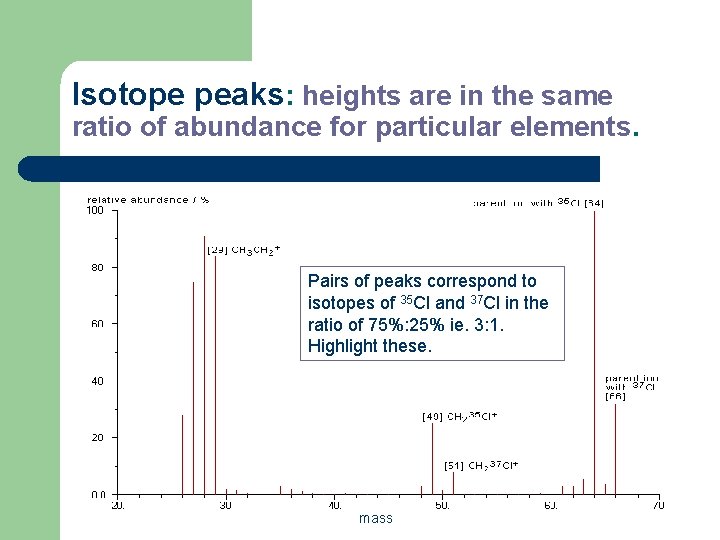
Isotope peaks: heights are in the same ratio of abundance for particular elements. Pairs of peaks correspond to isotopes of 35 Cl and 37 Cl in the ratio of 75%: 25% ie. 3: 1. Highlight these. mass

Now it’s over to you! Do activity WM 4: use accurate Mr values, isotope peaks and a database to lead you to the formula of salicylic acid. It shows you how chemists use fragmentation patterns to deduce or confirm a molecular structure. l Do assignments 1 and 2 C. S. p 110 -111 l Do ‘Problems for 6. 5’ on mass spectrometry, C. I. , p 145 -146. l
 Revenue streams example business model canvas
Revenue streams example business model canvas Business model canvas tripadvisor
Business model canvas tripadvisor Principles of instrumental analysis chapter 1 problem 1qp
Principles of instrumental analysis chapter 1 problem 1qp Electronic energy level
Electronic energy level Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton
Tư thế worm breton Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống