With the person next to you answer the





























- Slides: 60

With the person next to you, answer the following questions, which are based on your homework: 1. What types of nutrients are found in foods? 1. What makes a food ‘nutritious? ’ 1. What is your definition of a ‘nutrient? ’

What are nutrients? ? ? n Definition: n Nutrients are the components in foods that organisms use to survive and grow.

Unit 2 Nutrients of Life

Key Questions n 1. Why do organisms need/use nutrients? n 2. What are organic vs. inorganic compounds? n 3. What are some characteristics of certain nutrients? n 4. What are enzymes and how do they function? n 5. What factors affect the rate of enzyme action? n 6. What reactions do nutrients undergo?

Why do Organisms Need Nutrients? n Organisms must take in (ingest) essential nutrients from the environment and change them into a form that they can use. n Research has shown that good nutrition can help lower people’s risk for many chronic diseases (heart disease, stroke, diabetes) according to the CDC

1. Why do Organisms Need Nutrients? 1. Energy 2. Growth 3. Repair 4. Chemical Reactions 5. Regulation

n There are different types of nutrients – organic and inorganic – both are found in cells and allow them to perform reactions to maintain life.

Nutrition Song (4: 02)!!! n https: //www. youtube. com/watch? v=6 fh. SGWdbm 9 g

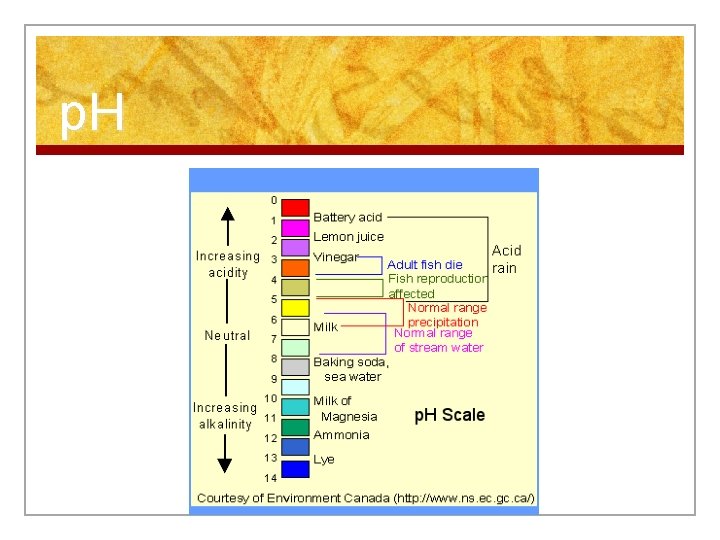
2. What are Organic vs. Inorganic Compounds? n Inorganic compounds: n Do not have BOTH carbon and hydrogen n Examples: n Water n Acids (p. H is 1 -6) n Bases (p. H is 8 -14) n Salts n Minerals (ex: calcium)

p. H

What are Organic vs. Inorganic Compounds? n Organic compounds: n Contain both carbon and hydrogen. n Types: n 1. Carbohydrates n 2. Lipids n 3. Proteins n 4. Nucleic Acids, and Vitamins

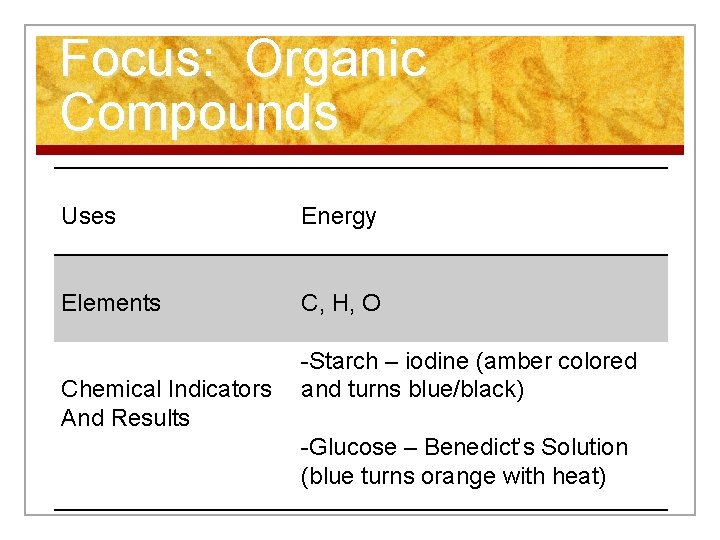
Focus: Organic Compounds a. Carbohydrates (Sugars) Examples Starch Glucose Cellulose Building Blocks Simple Sugars

Focus: Organic Compounds Uses Energy Elements C, H, O -Starch – iodine (amber colored Chemical Indicators and turns blue/black) And Results -Glucose – Benedict’s Solution (blue turns orange with heat)

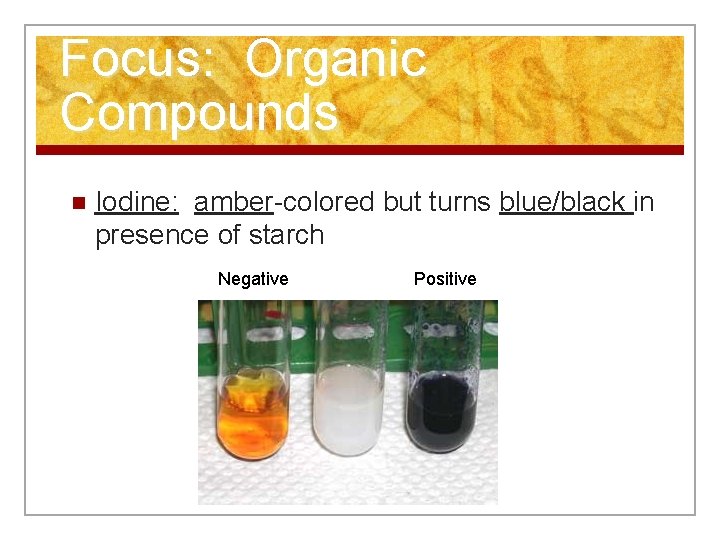
Focus: Organic Compounds n Iodine: amber-colored but turns blue/black in presence of starch Negative Positive

Focus: Organic Compounds n Benedict’s Solution: Blue-colored and turns orange in the presence of glucose when heated

Focus: Organic Compounds n The sugars we eat are very complex – they need to be broken down into simple sugars (like glucose) so that they can fit into our cells. n Starch is a HUGE sugar, and glucose is a small sugar!


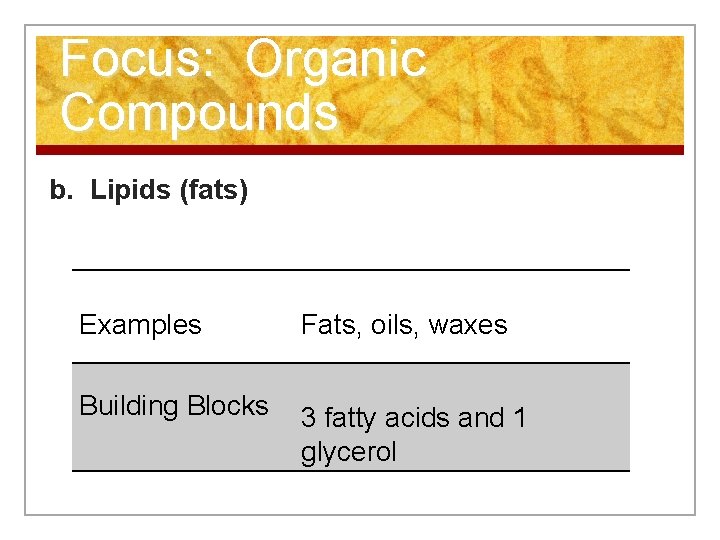
Focus: Organic Compounds b. Lipids (fats) Examples Fats, oils, waxes Building Blocks 3 fatty acids and 1 glycerol

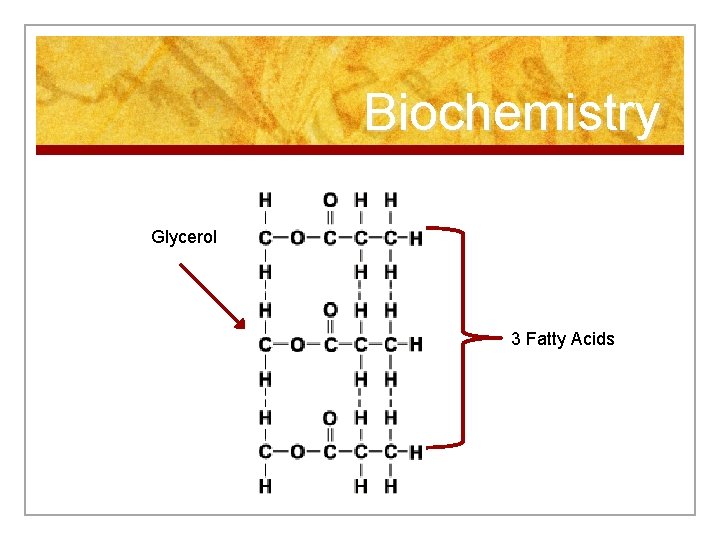
Biochemistry Glycerol 3 Fatty Acids

Biochemistry n. Examine a leaf!!! n Why does it feel waxy? ? ?

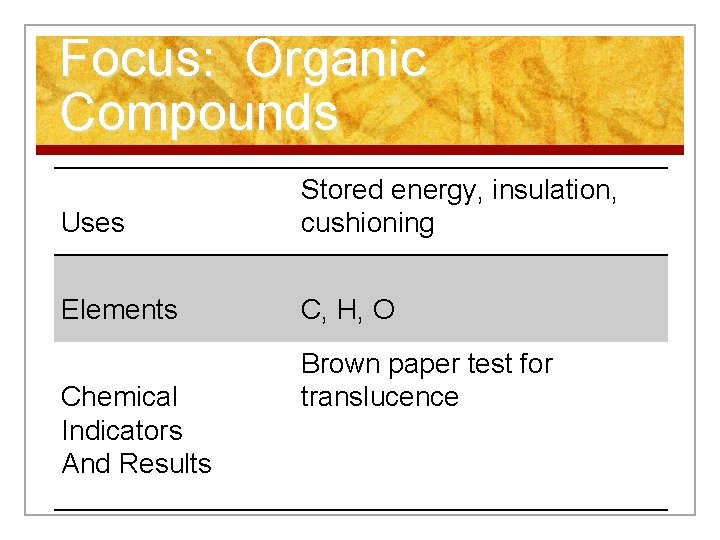
Focus: Organic Compounds Uses Stored energy, insulation, cushioning Elements C, H, O Chemical Indicators And Results Brown paper test for translucence

Brown Paper Test

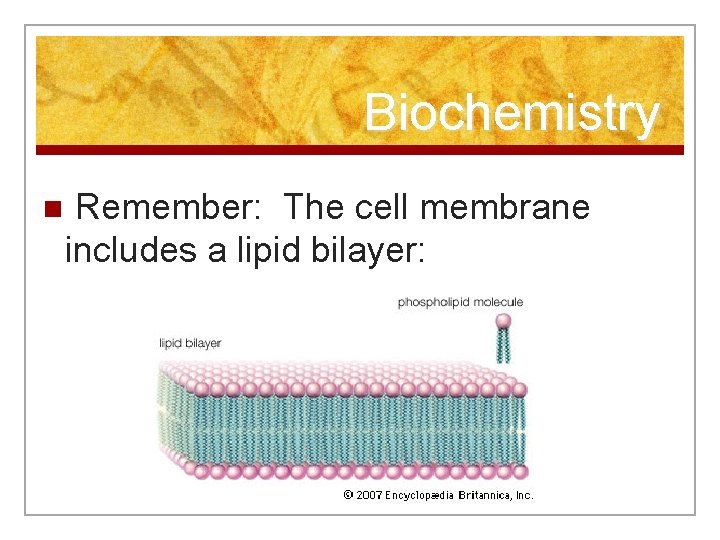
Biochemistry n Remember: The cell membrane includes a lipid bilayer:

Biochemistry n FYI – Cholesterol n Soft, waxy substance made by our bodies in the liver and from the animal foods that we eat (plant foods do not contain cholesterol) n Transported in the blood and used for body functions (production of hormones and vitamin D) n Combines with protein and fatty acids to form HDL – remove cholesterol from cells of body and LDL – ‘bad cholesterol (can stick together to form plaque on walls of blood vessels)

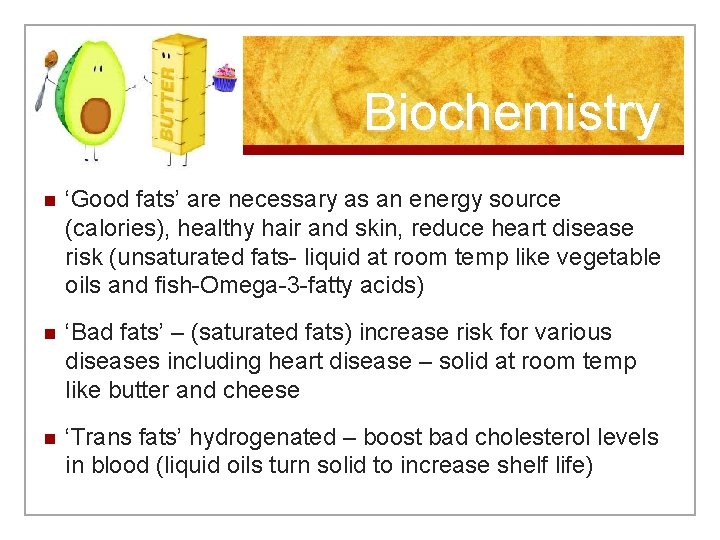
Biochemistry n ‘Good fats’ are necessary as an energy source (calories), healthy hair and skin, reduce heart disease risk (unsaturated fats- liquid at room temp like vegetable oils and fish-Omega-3 -fatty acids) n ‘Bad fats’ – (saturated fats) increase risk for various diseases including heart disease – solid at room temp like butter and cheese n ‘Trans fats’ hydrogenated – boost bad cholesterol levels in blood (liquid oils turn solid to increase shelf life)

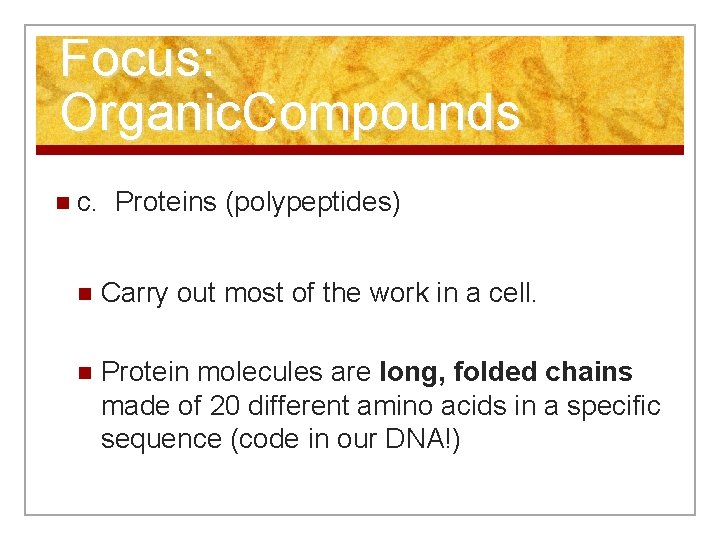
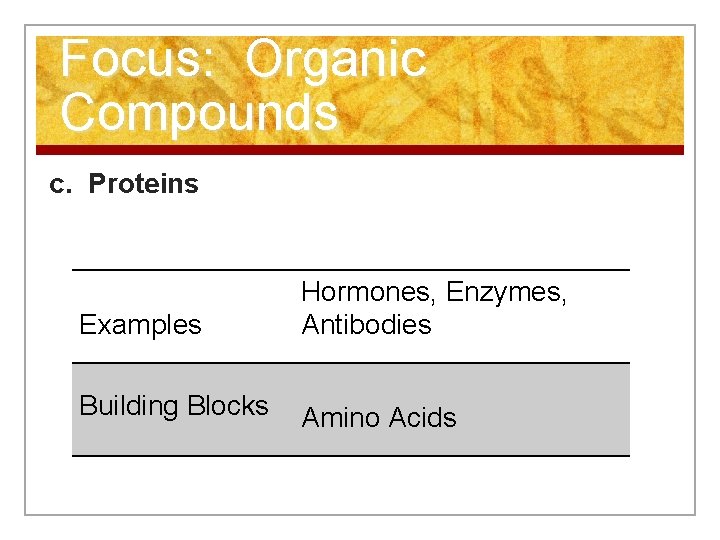
Focus: Organic. Compounds n c. Proteins (polypeptides) n Carry out most of the work in a cell. n Protein molecules are long, folded chains made of 20 different amino acids in a specific sequence (code in our DNA!)

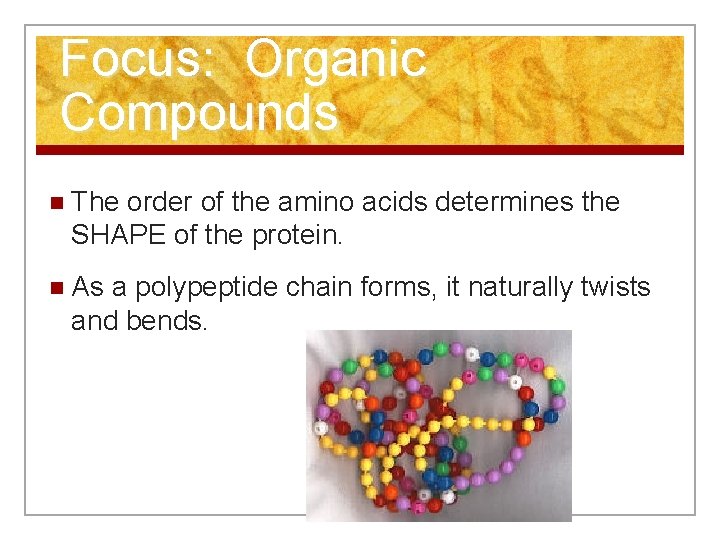
Focus: Organic Compounds n The order of the amino acids determines the SHAPE of the protein. n As a polypeptide chain forms, it naturally twists and bends.

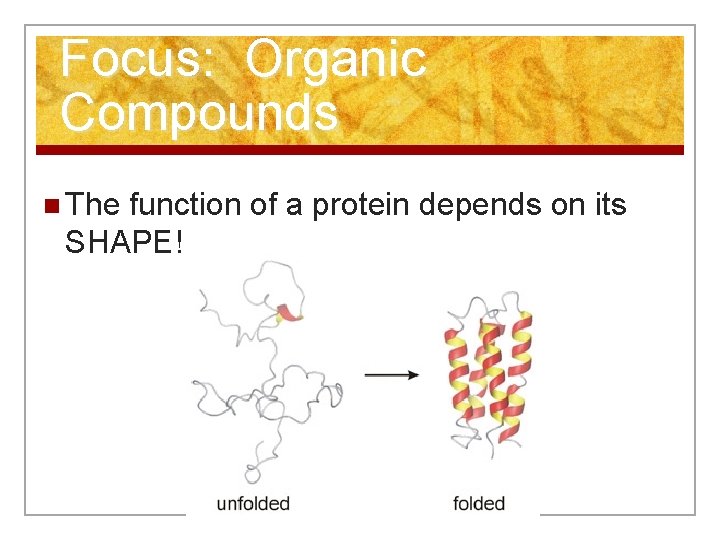
Focus: Organic Compounds n The function of a protein depends on its SHAPE!

Focus: Organic Compounds c. Proteins Examples Hormones, Enzymes, Antibodies Building Blocks Amino Acids

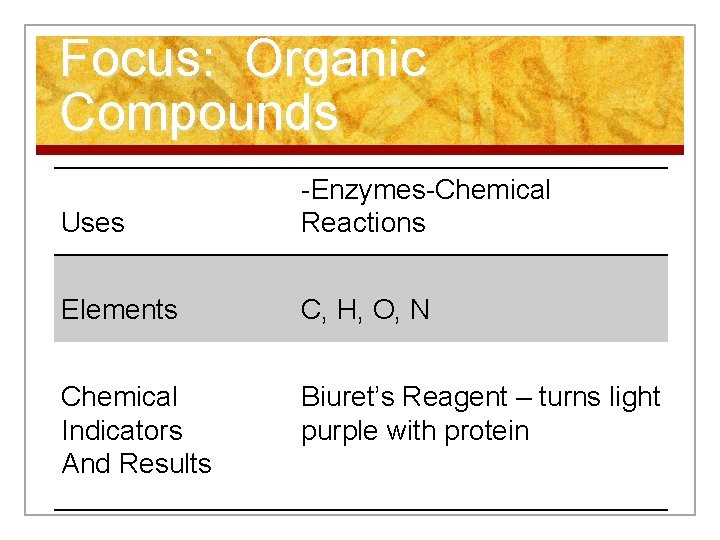
Focus: Organic Compounds Uses -Enzymes-Chemical Reactions Elements C, H, O, N Chemical Indicators And Results Biuret’s Reagent – turns light purple with protein

Focus: Organic Compounds n Biuret’s Reagent - turns light purple in the presence of protein.

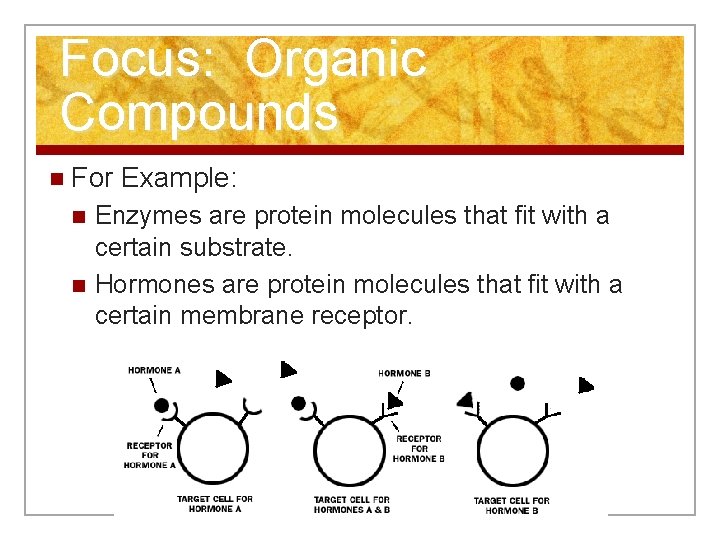
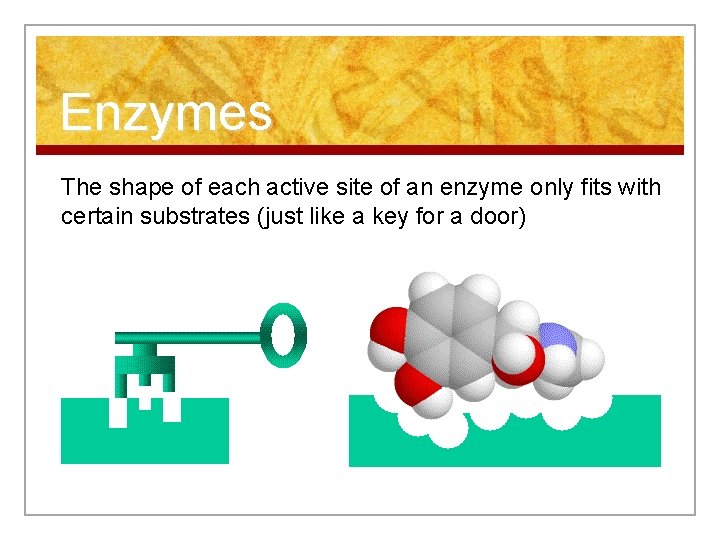
Focus: Organic Compounds n For Example: n n Enzymes are protein molecules that fit with a certain substrate. Hormones are protein molecules that fit with a certain membrane receptor.

Proteins !!! n http: //publications. nigms. nih. gov/structlife/chapter 1. html

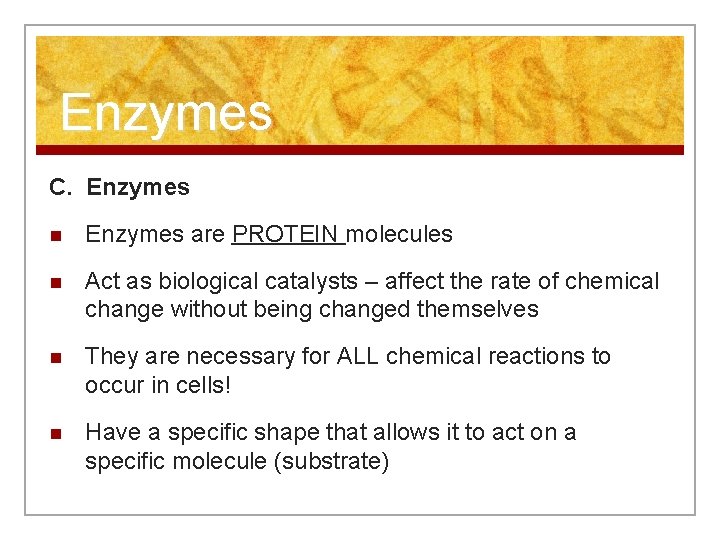
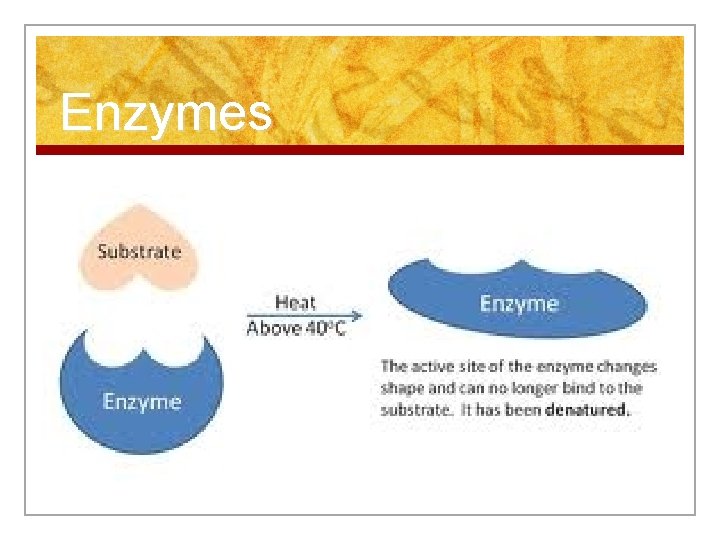
Enzymes C. Enzymes n Enzymes are PROTEIN molecules n Act as biological catalysts – affect the rate of chemical change without being changed themselves n They are necessary for ALL chemical reactions to occur in cells! n Have a specific shape that allows it to act on a specific molecule (substrate)

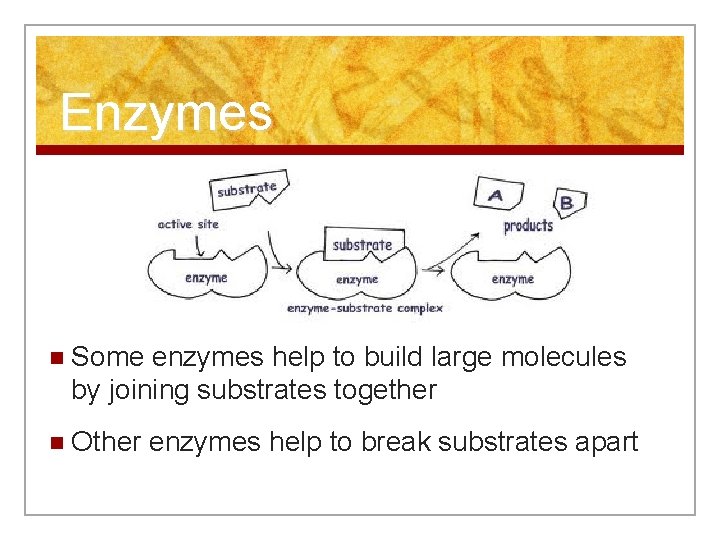
Enzymes n Some enzymes help to build large molecules by joining substrates together n Other enzymes help to break substrates apart

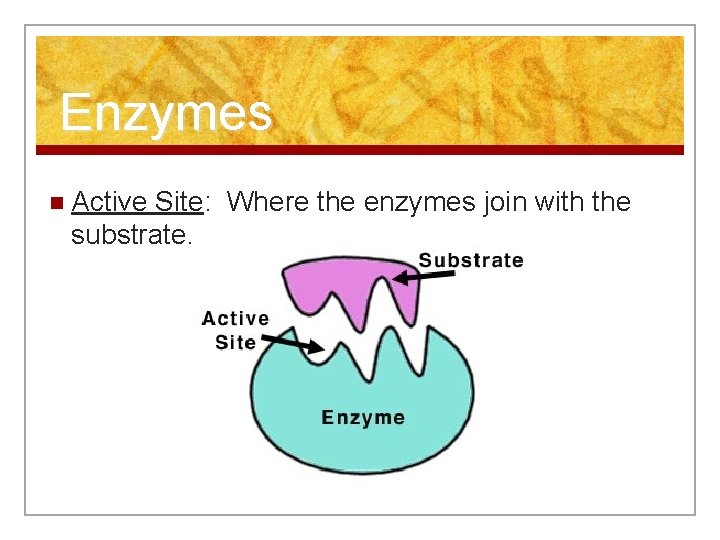
Enzymes n Active Site: Where the enzymes join with the substrate.

Thought Question n Why might enzyme action be referred to as the ‘Lock and Key Model’ for enzyme action?

Enzymes The shape of each active site of an enzyme only fits with certain substrates (just like a key for a door)

Enzyme Animations n http: //www. kscience. co. uk/animations/anim_2. htm

Enzymes n The rate at which enzymes work can be influenced by internal factors (inside your body) such as p. H and temperature.

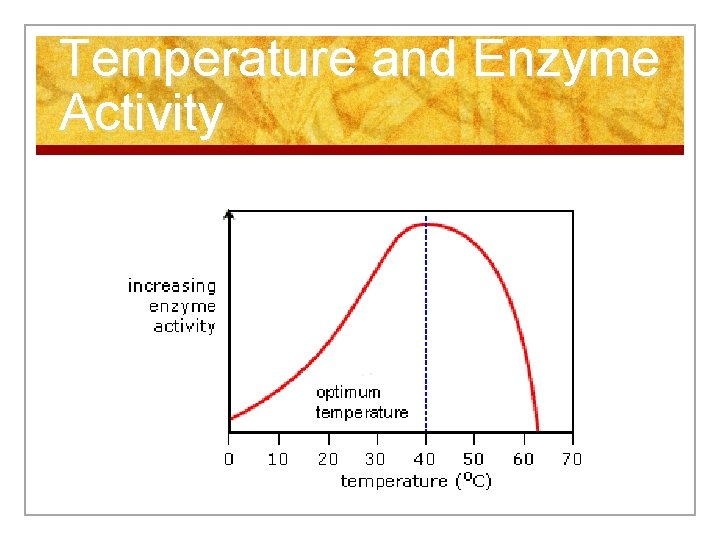
Enzymes n 1. Temperature n At high or low temperatures, enzymes may denature – enzyme changes shape, so it does not fit with the substrate n The reaction may slow down or stop

Enzymes

Temperature and Enzyme Activity


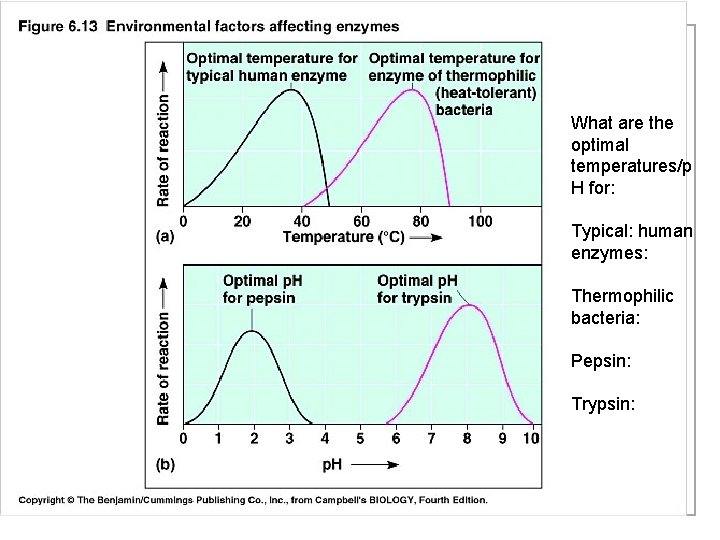
Enzymes n The optimum (best) temperature for enzymes depends on the organism! n Enzymes found in humans work best at 98. 6 degrees F n Those found in dogs work best at 101 degrees F

Enzymes n 2. p. H n The p. H an enzyme works best in is different for each enzyme.

What are the optimal temperatures/p H for: Typical: human enzymes: Thermophilic bacteria: Pepsin: Trypsin:

Lactose Intolerance Activity

Question – Lactose Intolerance n A lactose intolerant student fixed himself a cup of hot chocolate. n He added the hot chocolate mix, milk, and lactaid and placed the mixture in the microwave for 2 minutes. n He drank the hot chocolate while watching his favorite TV show and not soon after, he was running to the bathroom with awful cramps. n He was so confused – what happened? ? ?

Lactose Intolerance n Interesting fact! n People with mild intolerance can usually have yogurt because the live cultures (bacteria!) break down much of the lactose into glucose and galactose – simple sugars that are easier to digest.

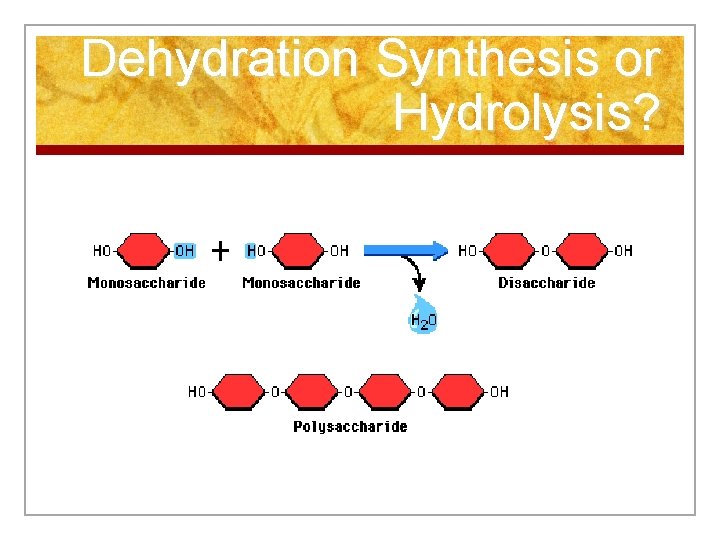
D. Reactions in Cells n Chemical reactions are occur constantly in cells and they require enzymes! n These reactions allow cells to build new molecules and break down others in order to maintain homeostasis. n The major reactions are called synthesis and hydrolysis.

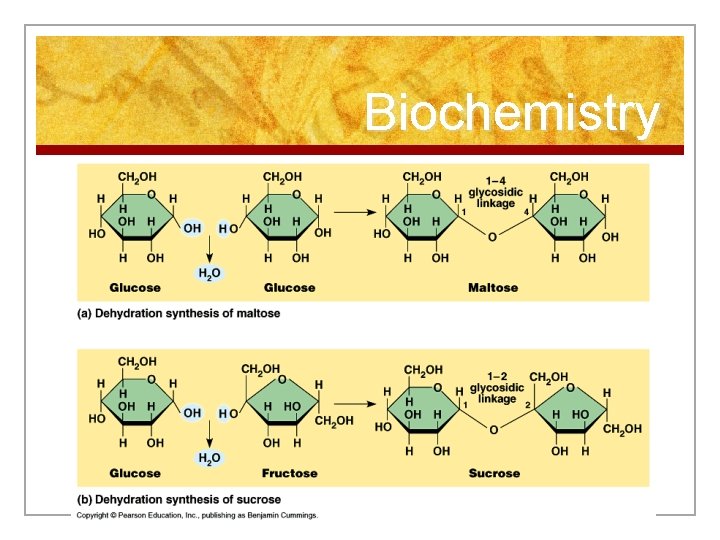
Reactions n 1. Synthesis n n n Building larger molecules from smaller ones with the use of enzymes When joining the pieces together, water is removed When small nutrients enter a cell, the cell will use them as building blocks in the synthesis of compounds necessary for life

Biochemistry

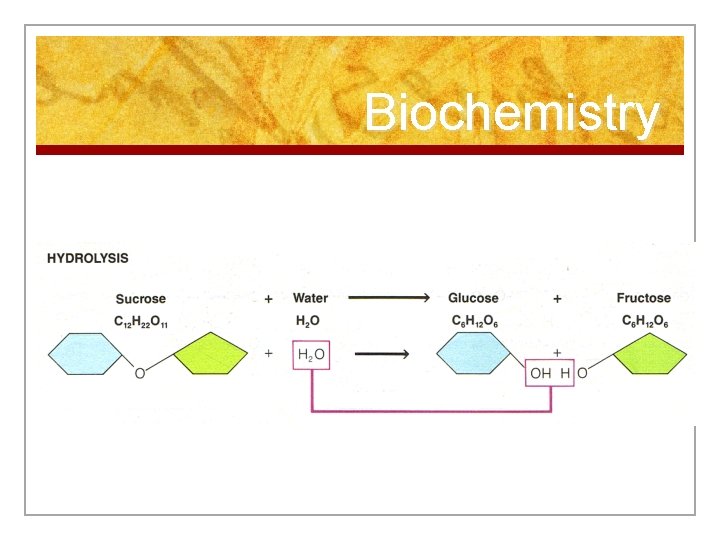
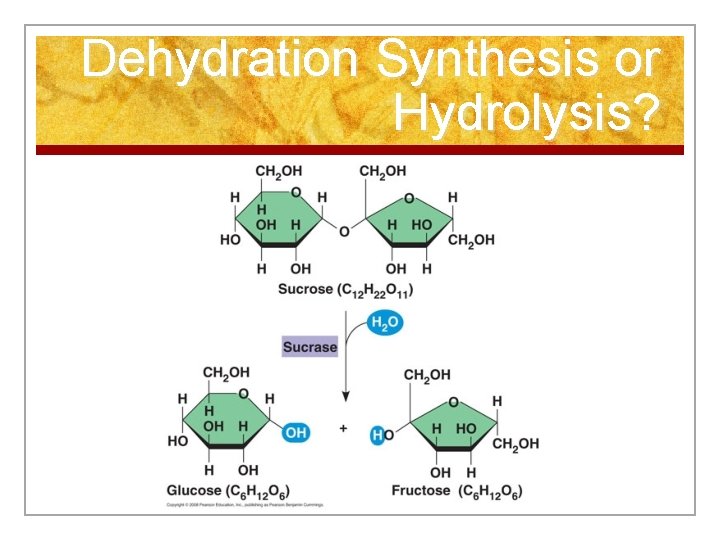
Reactions 2. Hydrolysis (Break Down) n Breaking down larger molecules into smaller materials by adding water with the use of enzymes n Occurs in digestion

Biochemistry

Biochemistry n PRACTICE: n Hydrolysis or Dehydration Synthesis?

Dehydration Synthesis or Hydrolysis?

Dehydration Synthesis or Hydrolysis?

Dehydration Synthesis or Hydrolysis?

Applicable NYS Standards n 1. 2 h, 2. 1 I, 5. 1 c, 5. 1 f, 5. 1 g