WIPO National Workshop on Intellectual Property for Diplomats



















- Slides: 29

WIPO National Workshop on Intellectual Property for Diplomats Sana’a, Republic of Yemen 20 -21 March 2007 The TRIPS Agreement and Public Health Roger Kampf WTO Secretariat 1

I. Overview: Relevant TRIPS Provisions Transition Periods 2

TRIPS Provisions of Direct Relevance to Public Health • • TRIPS Objectives and Principles Patents (incl. compulsory licences) Exhaustion Protection of Undisclosed Information • Competition • Measures against Counterfeiting • Transition periods 3

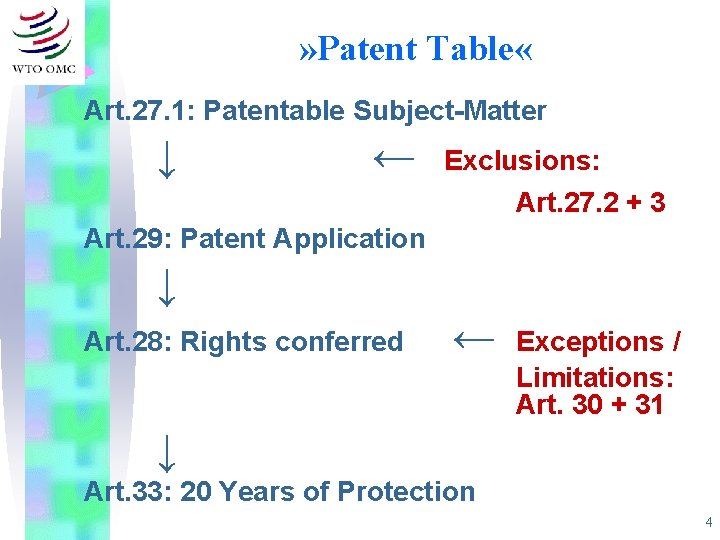
» Patent Table « Art. 27. 1: Patentable Subject-Matter ↓ ← Exclusions: Art. 27. 2 + 3 Art. 29: Patent Application ↓ Art. 28: Rights conferred ← Exceptions / Limitations: Art. 30 + 31 ↓ Art. 33: 20 Years of Protection 4

Article 30: Exceptions • Exceptions may be provided if they – are limited – do not unreasonably conflict with normal exploitation of the patent and – do not unreasonably prejudice the legitimate interests of the patent owner, taking account of the legitimate interests of third parties • Examples in national legislation: – Experimental use – Use to develop test data required to obtain marketing approval 5

Article 31: Compulsory Licences • Use of invention without authorization by right holder: – government use – use by third parties • not limited to specific purposes • must meet certain conditions, including: – prior efforts to obtain voluntary licence exception: public non-commercial use or national emergency – predominantly for supply of domestic market exception: adjudicated anti-competitive practices – adequate remuneration paid to patent owner ⇒ Doha Declaration / Para. 6 System 6

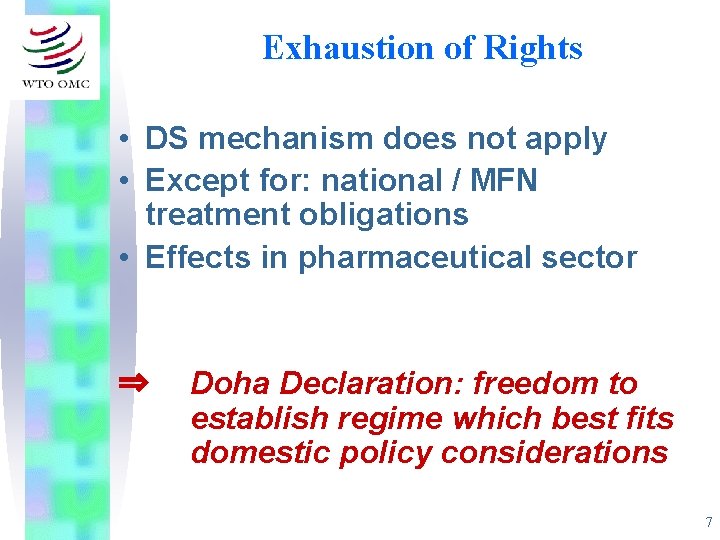
Exhaustion of Rights • DS mechanism does not apply • Except for: national / MFN treatment obligations • Effects in pharmaceutical sector ⇒ Doha Declaration: freedom to establish regime which best fits domestic policy considerations 7

Article 39. 3: Data Exclusivity • Members are obliged to protect test data against: – unfair commercial use when: • marketing approval for pharmaceuticals or agrochemicals requires submission of undisclosed data; • new chemical entities are utilized; • origination of data involves considerable efforts; • information is not publicly available – disclosure, unless: • disclosure is necessary to protect the public • steps have been taken to protect the data against unfair commercial use 8

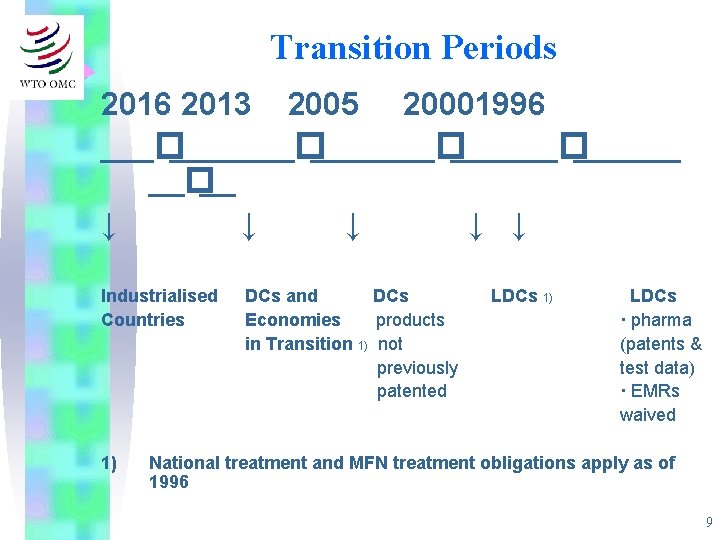
Transition Periods 2016 2013 2005 20001996 ___�_______�______ __�__ ↓ ↓ ↓ Industrialised Countries 1) DCs and DCs Economies products in Transition 1) not previously patented LDCs 1) LDCs · pharma (patents & test data) · EMRs waived National treatment and MFN treatment obligations apply as of 1996 9

II. The Doha Declaration on the TRIPS Agreement and Public Health 10

The Declaration: General Statements • Recognition of the gravity of the public health problems. . . , especially those resulting from HIV/AIDS, tuberculosis, malaria and other epidemics • Recognition that intellectual property protection is important for the development of new medicines and recognize concerns about its effects on prices • Agreement that the TRIPS Agreement does not and should not prevent Members from taking measures to protect public health. • Reaffirmation that TRIPS can and should be interpreted and implemented in a manner supportive of Members’ right to protect public health and, in particular, to promote access to medicines for all 11

Content of the Declaration • Guidance for disputes • Clarification compulsory licences: – right to grant – freedom to determine the grounds • Clarification of emergency situations: – right to determine what constitutes a national emergency of other circumstance of extreme urgency • Clarification of exhaustion: – freedom to establish own regime • Transfer of technology: – reaffirmation of Article 66. 2 commitment 12

Instructions for Further Work / Implementation • Find expeditious solution to difficulties of Members with insufficient / no manufacturing capacities in pharmaceutical sector in making effective use of compulsory licensing: ⇒ General Council Decision of August 2003 (WT/L/540); Chairman’s Statement (WT/GC/M/82, para. 29) ⇒ General Council Decision of 6 December 2005 (WT/L/641) - Protocol Amending the TRIPS Agreement; Chairman’s Statement (WT/GC/M/100) • Extend LDC transition period as regards protection and enforcement of patent rights / undisclosed information in the field of pharmaceuticals: ⇒ TRIPS Council Decision extends LDC transition period until 1/1/2016 (IP/C/25) ⇒ General Council Decision waives obligations under Article 70. 9 (WT/L/478) 13

III. August 2003 Decision: Implementation of Paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health 14

What is the problem ? • Members can issue compulsory licences for importation / domestic production • Concerns: – availability of supply from generic producers in third countries – mandatory patent protection for pharma products as from 2005 in exporting countries with important generic industry • Problem: Art. 31(f) requires production under compulsory licenses “predominantly for the supply of the domestic market of the Member“ 15

Solution: “Paragraph 6 System” (1) • August 2003 Decision is about addressing health problem in importing Member and legal problem in exporting Member • Decision consists of three waivers and calls for TRIPS amendment • General Council Chairman’s statement • Decision in effect since 30 August 2003, terminates when amendment replaces it for each Member 16

Three Distinct Waivers • Of 31(f) to exporting Members subject to conditions on transparency and safeguards: – scope and coverage: diseases and products – importing Members: • who is eligible • notification requirements • assessment of manufacturing capacities – notification requirements applying to exporting Members – safeguards against diversion • Of 31(h) to importing Members provided paid in exporting Member on same products • Of 31(f) to any LDC or developing country part of RTA where at least half LDCs 17

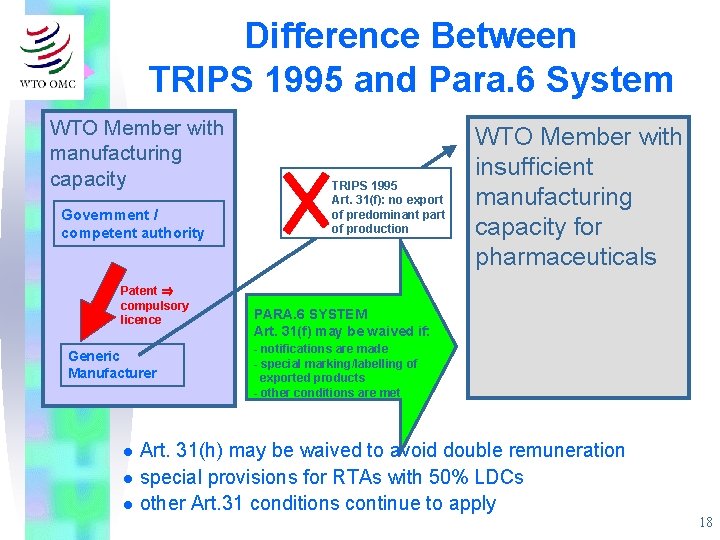
Difference Between TRIPS 1995 and Para. 6 System WTO Member with manufacturing capacity Government / competent authority Patent ⇒ compulsory licence Generic Manufacturer TRIPS 1995 Art. 31(f): no export of predominant part of production WTO Member with insufficient manufacturing capacity for pharmaceuticals PARA. 6 SYSTEM Art. 31(f) may be waived if: - notifications are made - special marking/labelling of exported products - other conditions are met Art. 31(h) may be waived to avoid double remuneration l special provisions for RTAs with 50% LDCs l other Art. 31 conditions continue to apply l 18

Other Elements • Facilitation of transfer of technology • Annual review substitutes review of waiver by the General Council • Preservation of existing flexibilities: – including under Article 31(f) – non-violation complaints not available 19

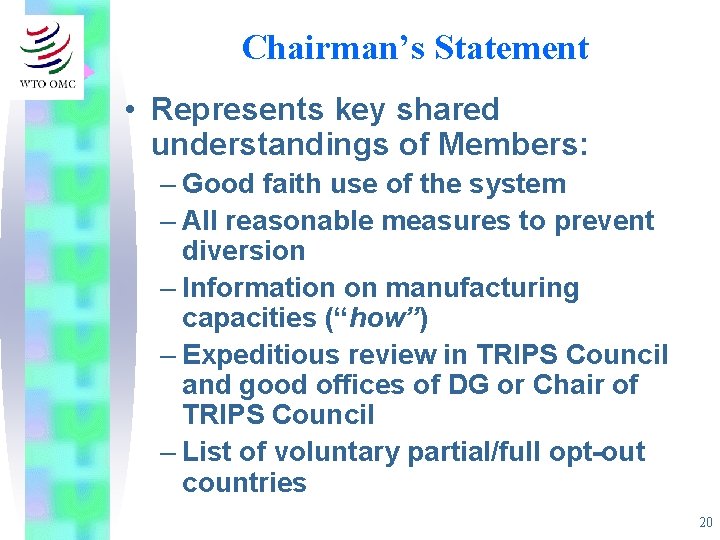
Chairman’s Statement • Represents key shared understandings of Members: – Good faith use of the system – All reasonable measures to prevent diversion – Information on manufacturing capacities (“how”) – Expeditious review in TRIPS Council and good offices of DG or Chair of TRIPS Council – List of voluntary partial/full opt-out countries 20

IV. December 2005 Decision: Protocol Amending the TRIPS Agreement 21

GC Decision WT/L/641 of 6 December 2005 • Basis: para. 11 of August 2003 Decision which instructed TRIPS Council to initiate work on amendment by end 2003 with a view to its adoption within 6 months • Adopts Protocol amending the TRIPS Agreement and submits it to Members for acceptance • Protocol open for acceptance until 1/12/2007 • Takes effect upon acceptance by two thirds of membership → Note that Paragraph 6 System as established under August 2003 Decision continues to apply until entry into force of amendment in a Member 22

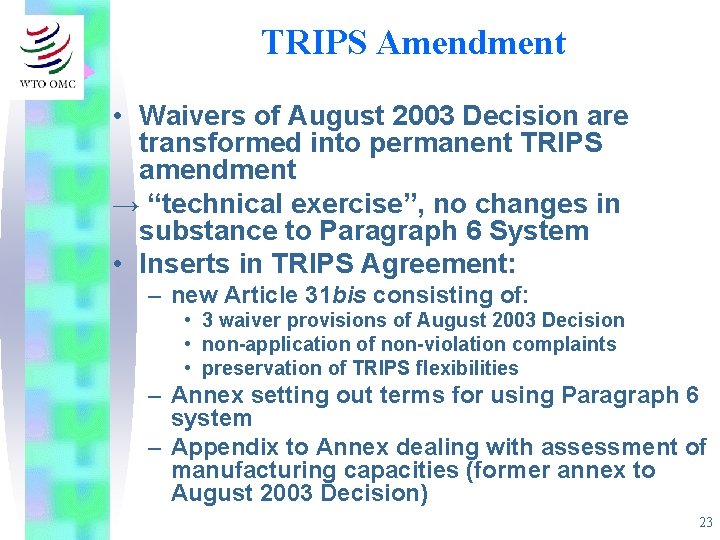
TRIPS Amendment • Waivers of August 2003 Decision are transformed into permanent TRIPS amendment → “technical exercise”, no changes in substance to Paragraph 6 System • Inserts in TRIPS Agreement: – new Article 31 bis consisting of: • 3 waiver provisions of August 2003 Decision • non-application of non-violation complaints • preservation of TRIPS flexibilities – Annex setting out terms for using Paragraph 6 system – Appendix to Annex dealing with assessment of manufacturing capacities (former annex to August 2003 Decision) 23

Statements by Chairman Recognition by MC • Chairman’s Statement: – re-read prior to adoption of GC Decision – updates list of full opt-out countries • Chairman’s Statement on non-violation complaints: amendment without prejudice to overall question of applicability of NVCs to TRIPS • Hong Kong MC in December 2005: Ministers welcome work on TRIPS amendment to implement August 2003 Decision 24

V. Final Remarks 25

Implementing Legislation in Exporting Members • Based on information shared in the TRIPS Council: – Norway, Canada, India, EC (in effect) – Korea – Switzerland (in process) • China also adopted implementing legislation in December 2005 26

Use of Paragraph 6 System • Possible reasons for absence of notifications so far: – generic medicines available outside patent system – legislative changes in exporting countries recent or not yet done – meant to address situation where nonpredominant limit proves restrictive – voluntary licences and reduction of prices offered by patent owners • Remember: system not to be used if – – local production voluntary licences no patents in exporting country not a WTO Member 27

Concluding Remarks • TRIPS forms part of the solution next to other important factors: infrastructure, national health systems, procurement regimes, import tariffs, etc. • Increased recognition of TRIPS flexibilities → but: need for each country to take the necessary steps at national level to avail itself of such flexibilities • TRIPS beyond Doha: new rules ? What role for DSU and FTAs ? 28

Some References • Doha Declaration on TRIPS and Public Health (WT/MIN(01)/DEC/2) • Decision on the implementation of paragraph 6 of the Doha Declaration on TRIPS and Public Health (WT/L/540 and Corr. 1) • Decision on an amendment to the TRIPS Agreement (Protocol) (WT/L/641) • Decision on extension of transition period for LDCs with respect to pharmaceutical products (IP/C/25) • Decision on general extension of transition period for LDCs (IP/C/40) 29
 Trade related aspects of intellectual property rights
Trade related aspects of intellectual property rights Secondary infringement
Secondary infringement Importance of intellectual property
Importance of intellectual property Intellectual property management definition
Intellectual property management definition Advantages of intellectual property
Advantages of intellectual property Example of intellectual property in business plan
Example of intellectual property in business plan Intellectual property in computer ethics
Intellectual property in computer ethics Right to intellectual property of teachers
Right to intellectual property of teachers Definition of ipr
Definition of ipr Incentive theory
Incentive theory Concept of intellectual property
Concept of intellectual property Valuation of ip
Valuation of ip Intellectual property rights
Intellectual property rights Intellectual property rights
Intellectual property rights Characteristics of intellectual property
Characteristics of intellectual property Discuss intellectual property frankly
Discuss intellectual property frankly Gaap accounting for intellectual property
Gaap accounting for intellectual property Intellectual property
Intellectual property Intellectual property statement
Intellectual property statement Intellectual property business plan example
Intellectual property business plan example Evalueserve competitors
Evalueserve competitors Discuss intellectual property frankly
Discuss intellectual property frankly At&t intellectual property
At&t intellectual property Wipo dl 101
Wipo dl 101 Wipo e learning
Wipo e learning Wipo e learning
Wipo e learning [email protected]
[email protected] Global brand database
Global brand database Global brand database
Global brand database Wipo patent drafting manual
Wipo patent drafting manual




















































