Why Water is Weird Chpt 2 4 p





- Slides: 12

Why Water is Weird Chpt. 2. 4 p. 72 - 78 ☂ How long can we live without food? ☂ How long can we live without water? ☂ So, why don’t we think water is amazing?

v. Did you know that water is The only substance that exists on Earth in large quantities in all three states? Less dense in its solid state (ice) than its liquid state so it floats on top of lakes in the winter instead of sinking (YAHOO for HOCKEY, SPEED SKATING, FIGURE SKATING!) Look at Table 2. 14 in your text – water is the weirdo substance in the group

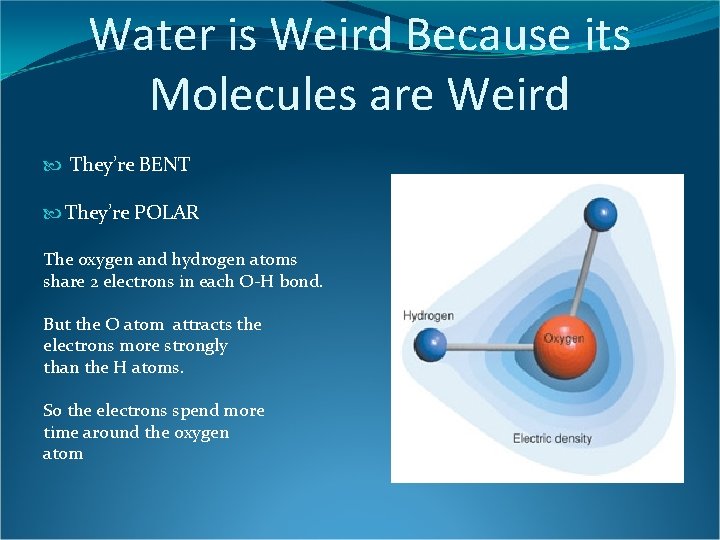
Water is Weird Because its Molecules are Weird They’re BENT They’re POLAR The oxygen and hydrogen atoms share 2 electrons in each O-H bond. But the O atom attracts the electrons more strongly than the H atoms. So the electrons spend more time around the oxygen atom

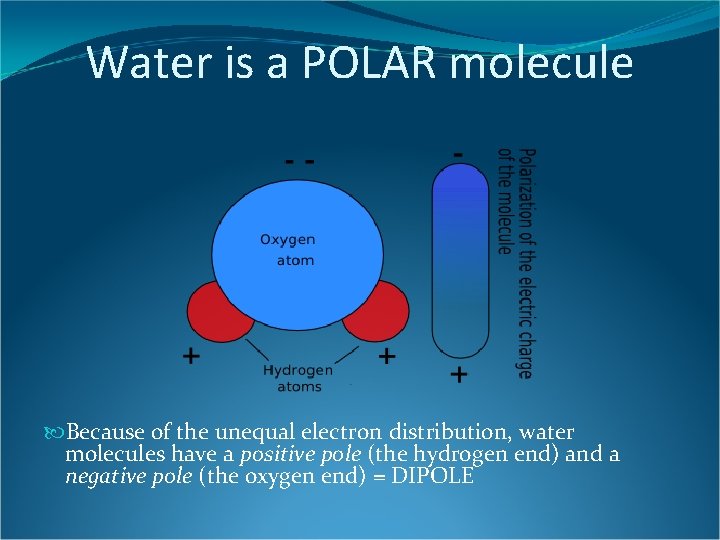
Water is a POLAR molecule Because of the unequal electron distribution, water molecules have a positive pole (the hydrogen end) and a negative pole (the oxygen end) = DIPOLE

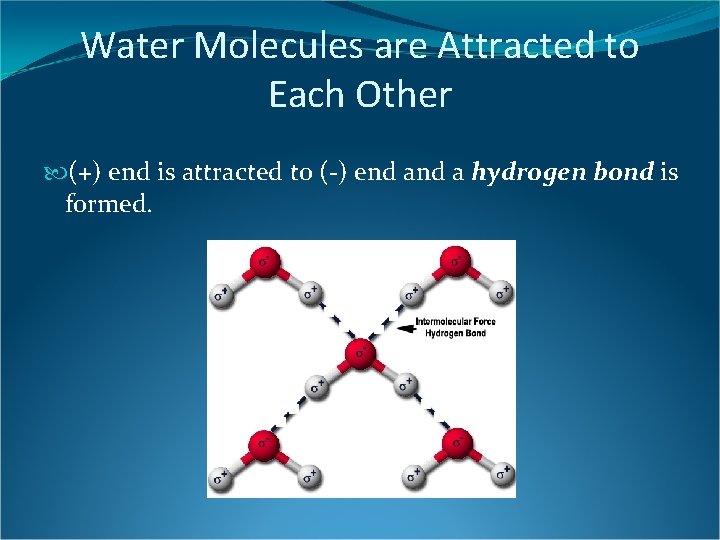
Water Molecules are Attracted to Each Other (+) end is attracted to (-) end a hydrogen bond is formed.

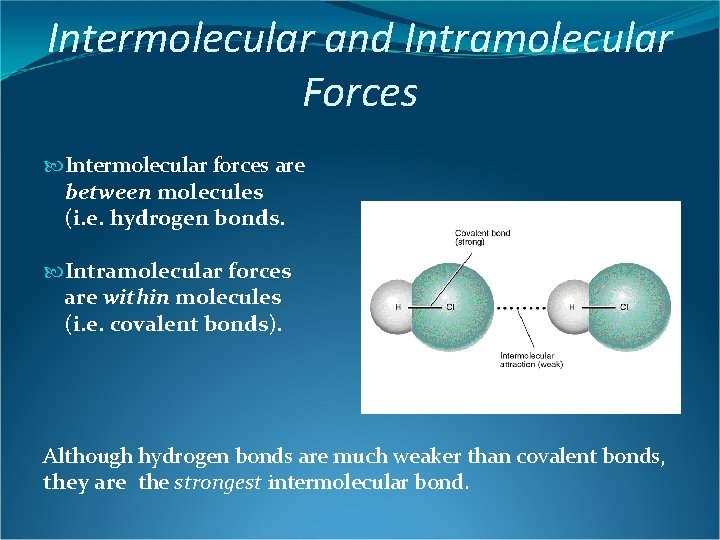
Intermolecular and Intramolecular Forces Intermolecular forces are between molecules (i. e. hydrogen bonds. Intramolecular forces are within molecules (i. e. covalent bonds). Although hydrogen bonds are much weaker than covalent bonds, they are the strongest intermolecular bond.

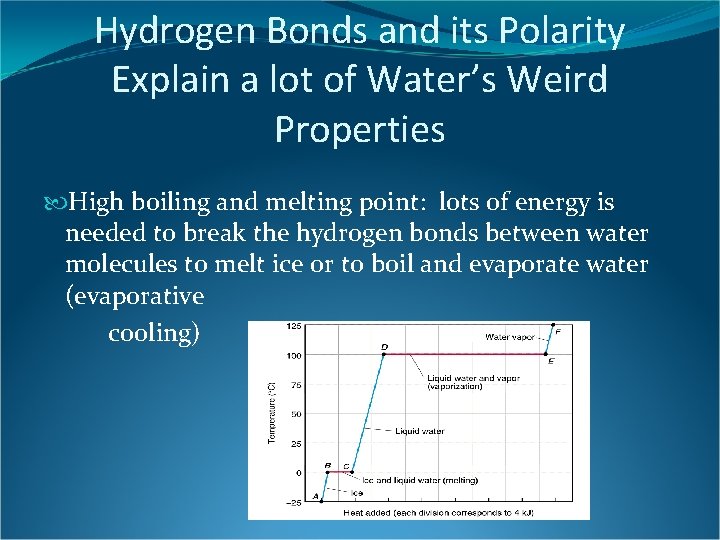
Hydrogen Bonds and its Polarity Explain a lot of Water’s Weird Properties High boiling and melting point: lots of energy is needed to break the hydrogen bonds between water molecules to melt ice or to boil and evaporate water (evaporative cooling)

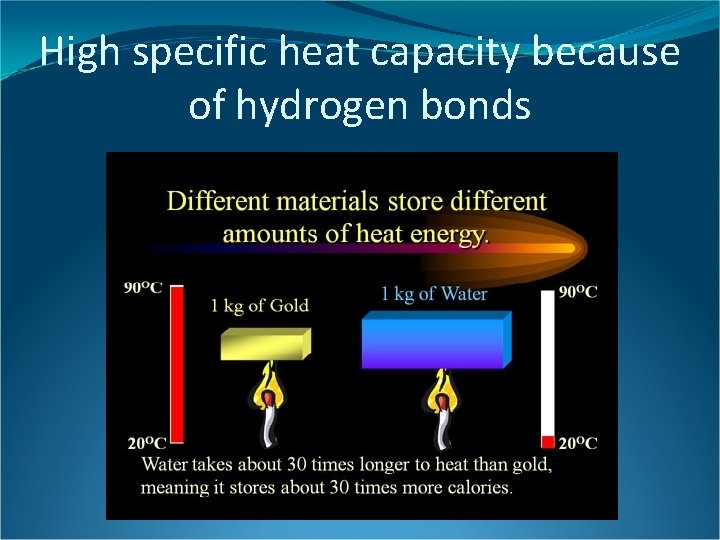
High specific heat capacity because of hydrogen bonds

Ice is less dense than water Water expands when it freezes because the polar molecules arrange themselves in an open pattern held together by hydrogen bonds.

High Surface Tension Water has high surface tension because the hydrogen bonds pull the molecules together into the smallest possible area.

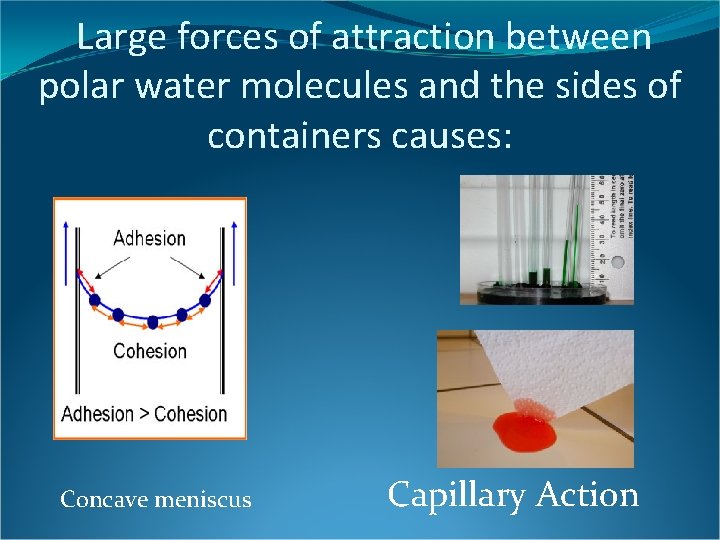
Large forces of attraction between polar water molecules and the sides of containers causes: Concave meniscus Capillary Action

Capillary action is the force that draws water up from roots to the leaves of tall trees