Why should we study chemistry in Life depends























- Slides: 74

Why should we study chemistry in

Life depends on chemistry! • When you eat food or inhale oxygen, your body uses these materials in chemical reactions that keep you alive. • Just as buildings are made from bricks, steel, glass, and wood, living things are made from chemical compounds. • Wouldn’t you want an architect to understand building materials? Same idea applies to geneticists, ecologists, zoologists, botanists, biologists, and etc.

• The study of chemistry begins with the basic unit of matter…the History • Greeks were first to try to explain chemical reactions • 400 BC: thought all matter composed of: – – Fire Earth Water Air • Democritus first used word “atomos”, meaning indivisible

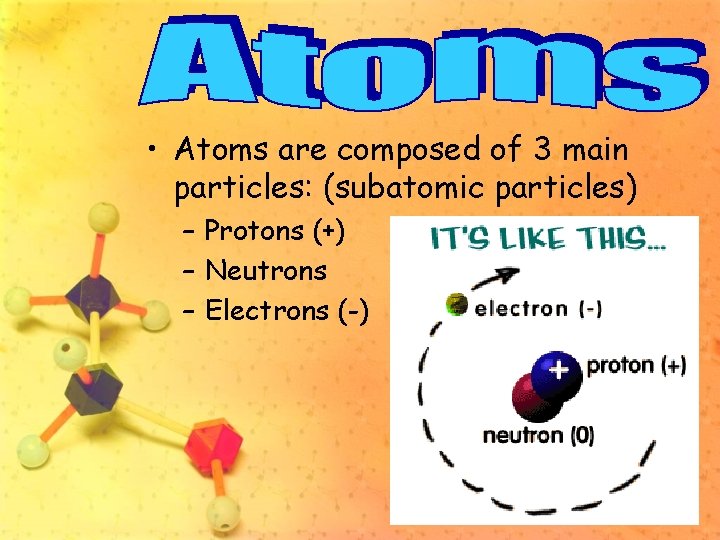
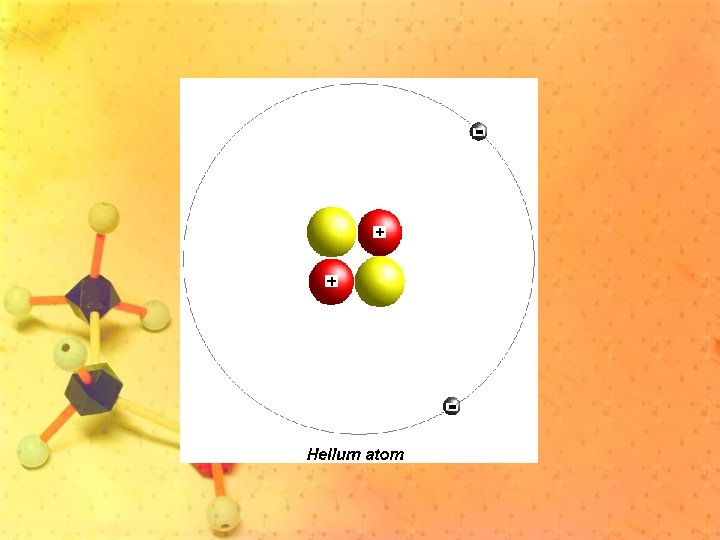
• Atoms are composed of 3 main particles: (subatomic particles) – Protons (+) – Neutrons – Electrons (-)

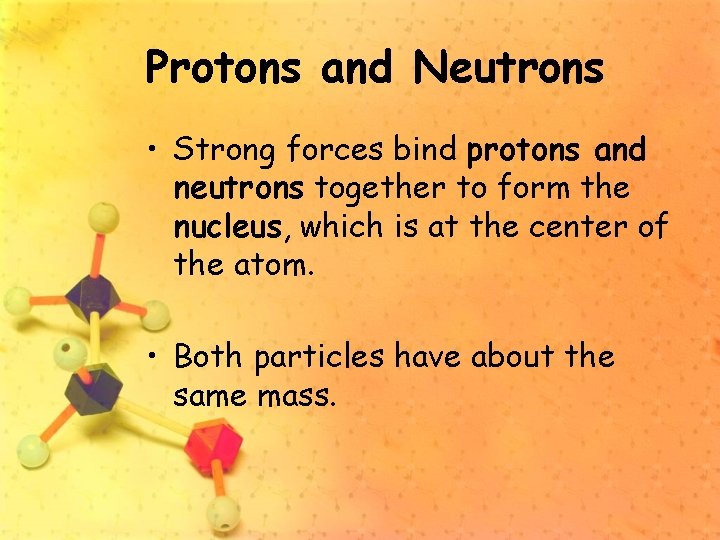
Protons and Neutrons • Strong forces bind protons and neutrons together to form the nucleus, which is at the center of the atom. • Both particles have about the same mass.

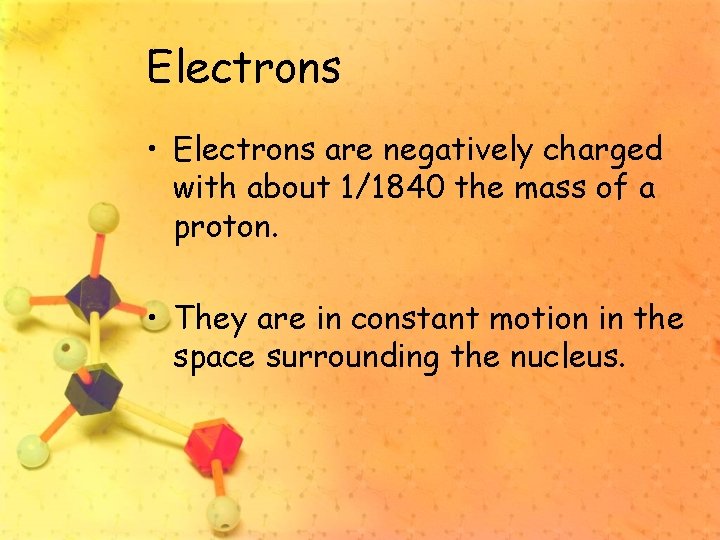
Electrons • Electrons are negatively charged with about 1/1840 the mass of a proton. • They are in constant motion in the space surrounding the nucleus.

• Atoms have equal numbers of electrons and protons. • Because these subatomic particles have equal but opposite charges, atoms are neutral.


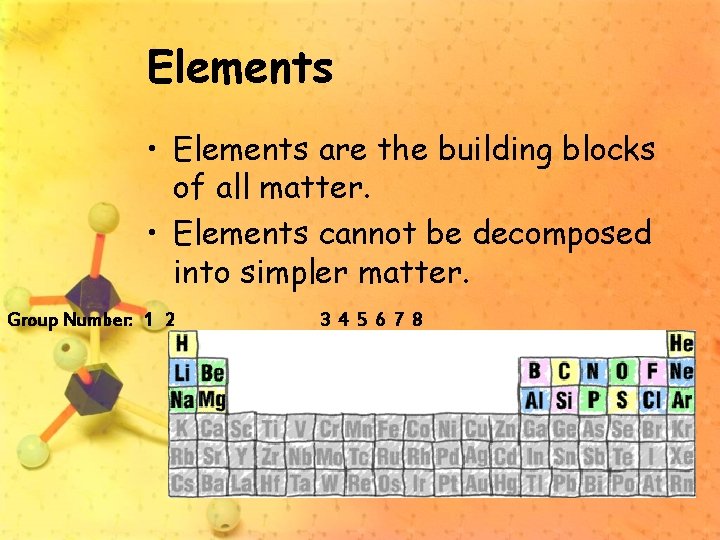
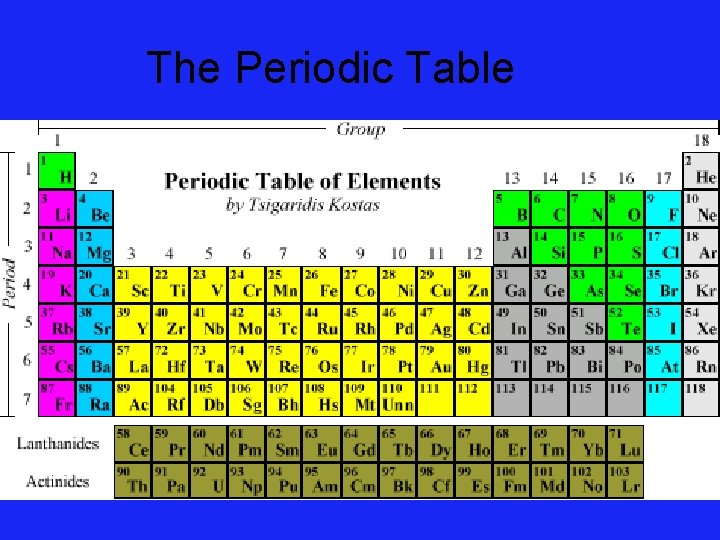
Elements • Elements are the building blocks of all matter. • Elements cannot be decomposed into simpler matter. Group Number: 1 2 3 4 5 6 7 8

The Elements • 110 known elements • 88 occur naturally The 110 elements form a plethora of compounds, just as 26 letters of the alphabet make a seemingly endless number of words.

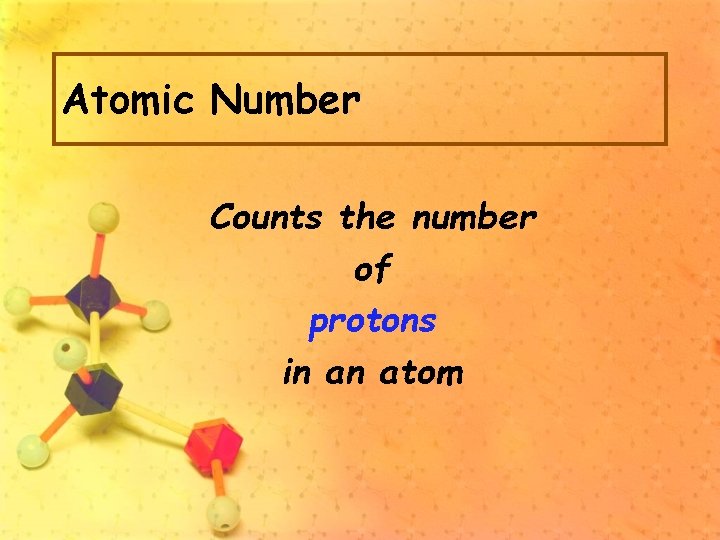
Atomic Number Counts the number of protons in an atom

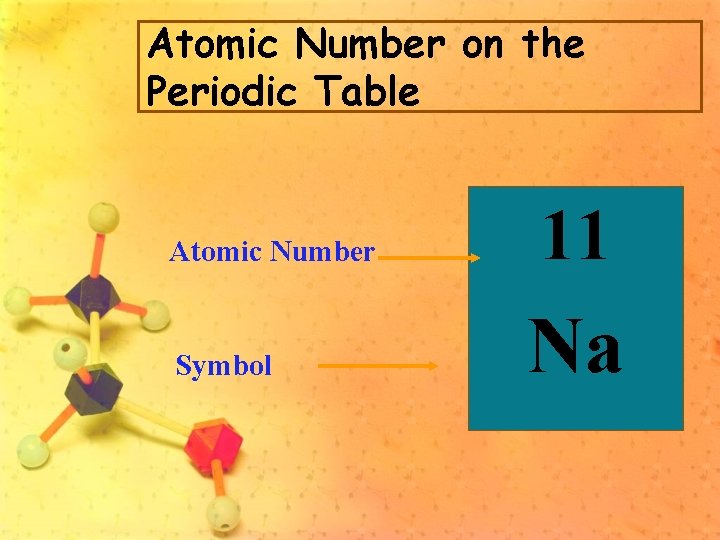
Atomic Number on the Periodic Table Atomic Number Symbol 11 Na

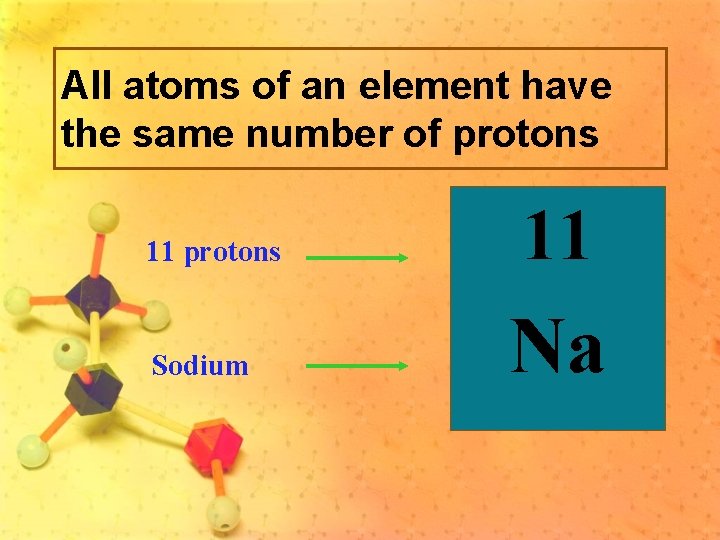
All atoms of an element have the same number of protons 11 protons Sodium 11 Na

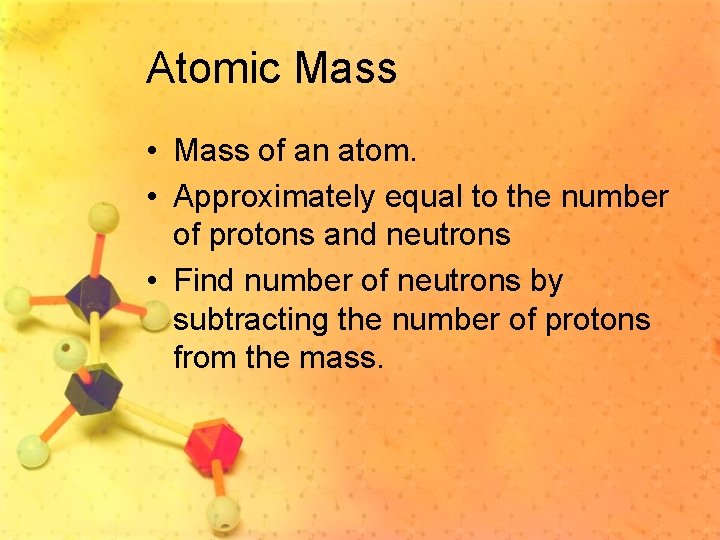
Atomic Mass • Mass of an atom. • Approximately equal to the number of protons and neutrons • Find number of neutrons by subtracting the number of protons from the mass.

Review: • An element's atomic number tells how many protons are in its atoms. • An element's mass number tells how many protons and neutrons are in its atoms.

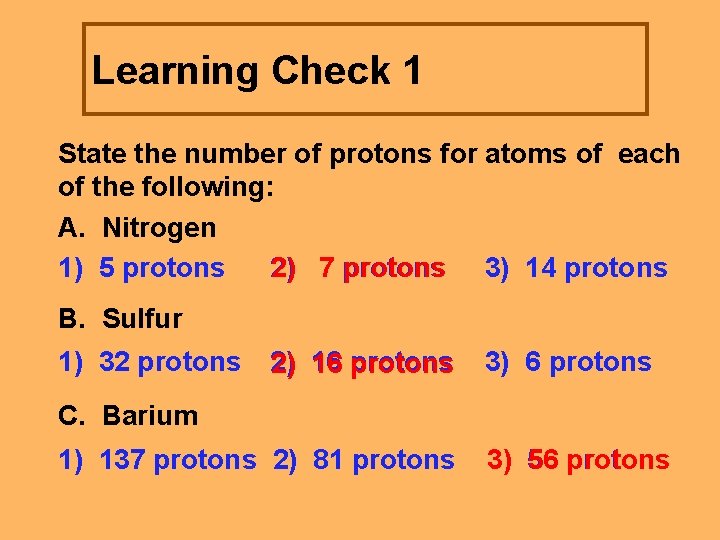
Learning Check 1 State the number of protons for atoms of each of the following: A. Nitrogen 1) 5 protons 2) 7 protons 3) 14 protons B. Sulfur 1) 32 protons 2) 16 protons 3) 6 protons C. Barium 1) 137 protons 2) 81 protons 3) 56 protons

The Periodic Table

Isotopes • Isotopes are atoms that have the same atomic number but different mass number. • Most elements have two or more isotopes. • Same chemical properties because the electron number does not change.

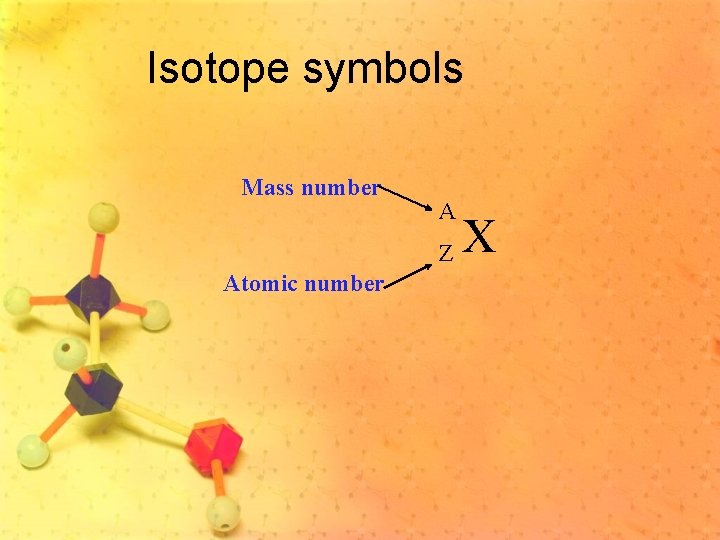
Isotope symbols Mass number A Z Atomic number X

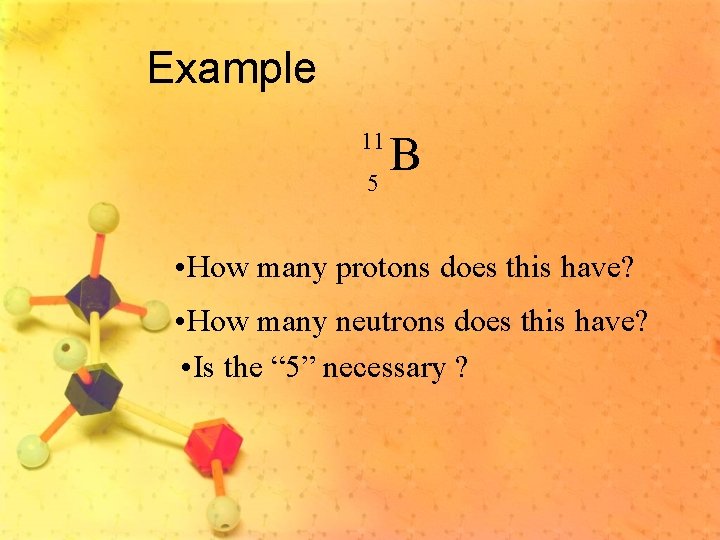
Example B 5 11 • How many protons does this have? • How many neutrons does this have? • Is the “ 5” necessary ?

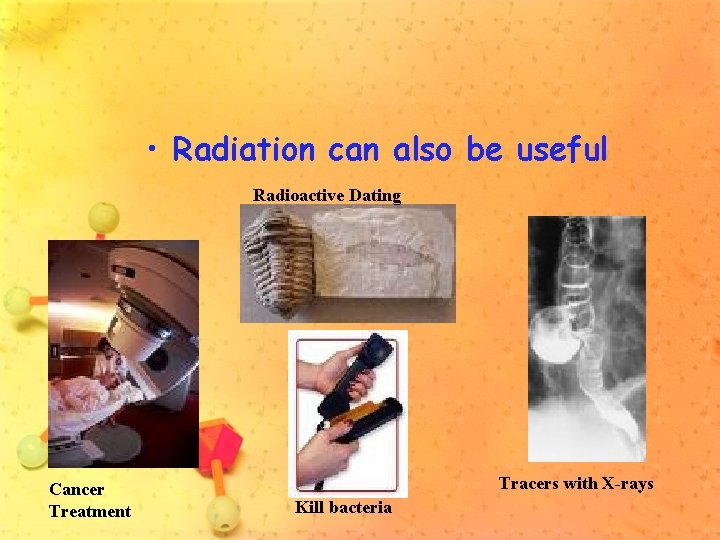
More about isotopes: • Some isotopes have unstable nuclei which break down over time. • They are called radioactive isotopes • Some radiation is harmful.

• Radiation can also be useful Radioactive Dating Cancer Treatment Tracers with X-rays Kill bacteria

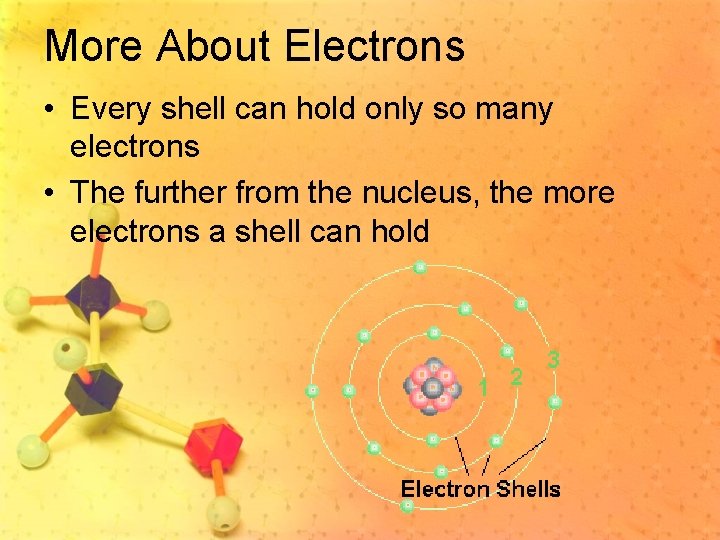
More About Atomic Structure • The center of the atom is called the nucleus. • Electrons live in something called shells. • Shells areas that surround the center of an atom. • A shell is sometimes called an orbital or energy level.

More About Electrons • Every shell can hold only so many electrons • The further from the nucleus, the more electrons a shell can hold

Valence Electrons • The electrons on the outside edge of the atom • This is where the action is- where bonding takes place • Atoms have no more than 8 valence electrons

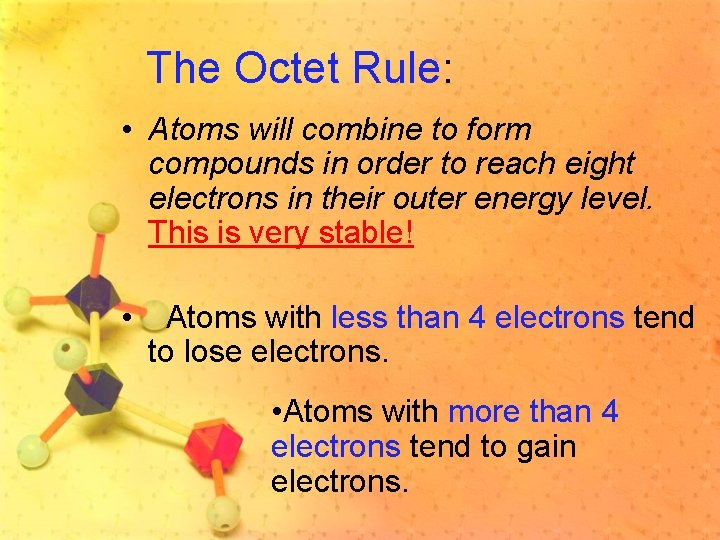
The Octet Rule: • Atoms will combine to form compounds in order to reach eight electrons in their outer energy level. This is very stable! • Atoms with less than 4 electrons tend to lose electrons. • Atoms with more than 4 electrons tend to gain electrons.

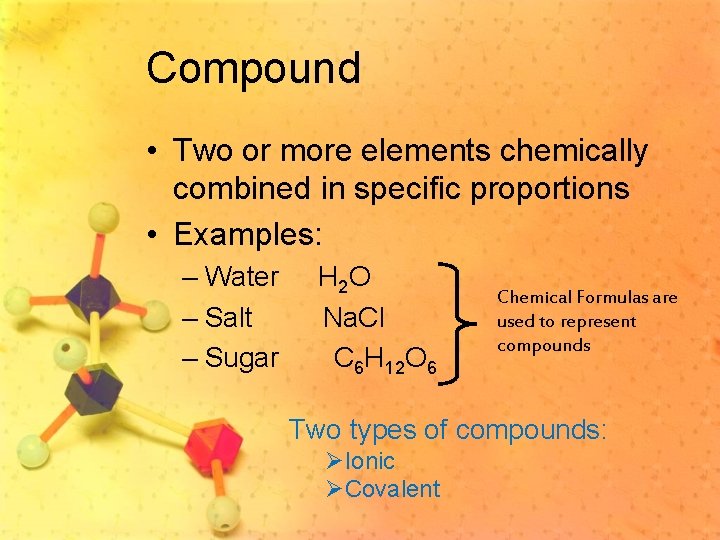
Compound • Two or more elements chemically combined in specific proportions • Examples: – Water – Salt – Sugar H 2 O Na. Cl C 6 H 12 O 6 Chemical Formulas are used to represent compounds Two types of compounds: ØIonic ØCovalent

Ionic Compounds • Form when electrons are transferred from one atom to another. Ions - Atoms with a net charge due to gaining or losing electrons – Gaining electrons gives an ion a negative charge – Losing electrons gives an ion a positive charge **If they have to choose, atoms would rather be stable (with a full “octet”) than neutral.

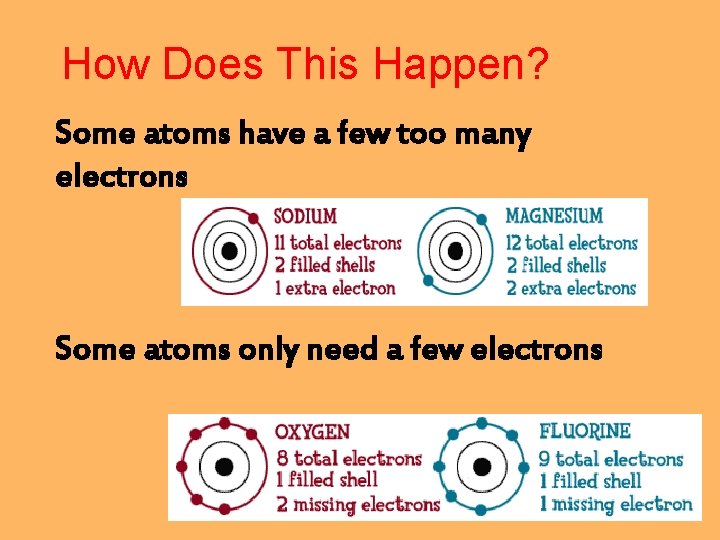
How Does This Happen? Some atoms have a few too many electrons Some atoms only need a few electrons

What do you do if you are a sodium (Na) atom with one extra electron? Go look for an atom that wants it!

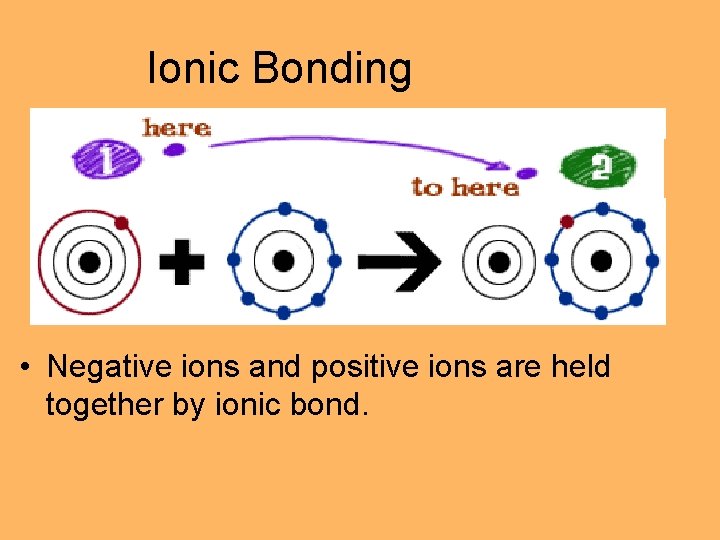
Ionic Bonding • Negative ions and positive ions are held together by ionic bond.

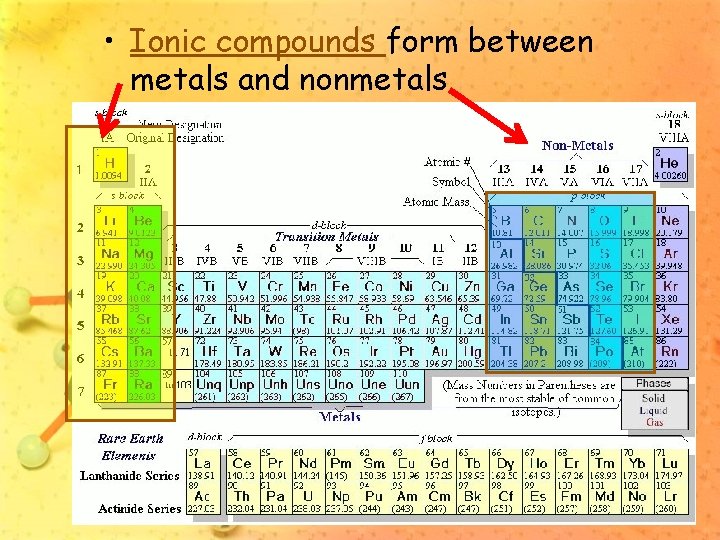
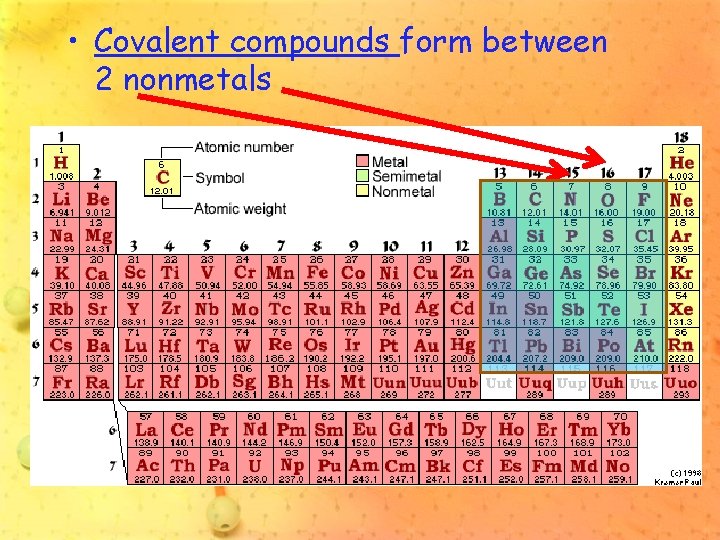
• Ionic compounds form between metals and nonmetals

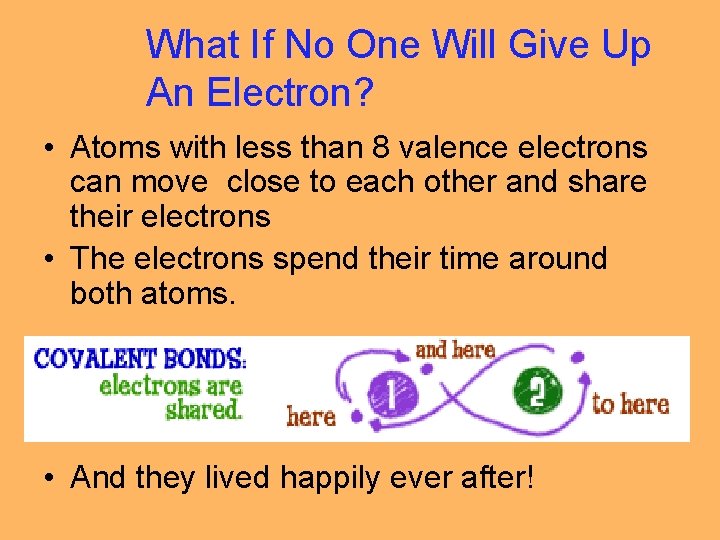
What If No One Will Give Up An Electron? • Atoms with less than 8 valence electrons can move close to each other and share their electrons • The electrons spend their time around both atoms. • And they lived happily ever after!

Covalent Bonds • Formed when a pair of electrons is shared between two atoms. • Sometimes the atoms share two pairs of electrons and form a double bond, or three pairs of electrons to form a triple bond. • Structures formed by covalent bonds are molecules.

• Covalent compounds form between 2 nonmetals

Van der Waals Forces • There are small attractive forces between all atoms • Help to hold molecules to each other – Ex: Gecko

Let’s summarize what we know! Why do compounds form? • Atoms are trying to get 8 valence electrons How do compounds form? • By ionic (e- transfer) or covalent (e- sharing) bonding How can you tell if a compound is ionic or covalent? • By the types of elements in the compound (ionic = M + M covalent = M + NM)

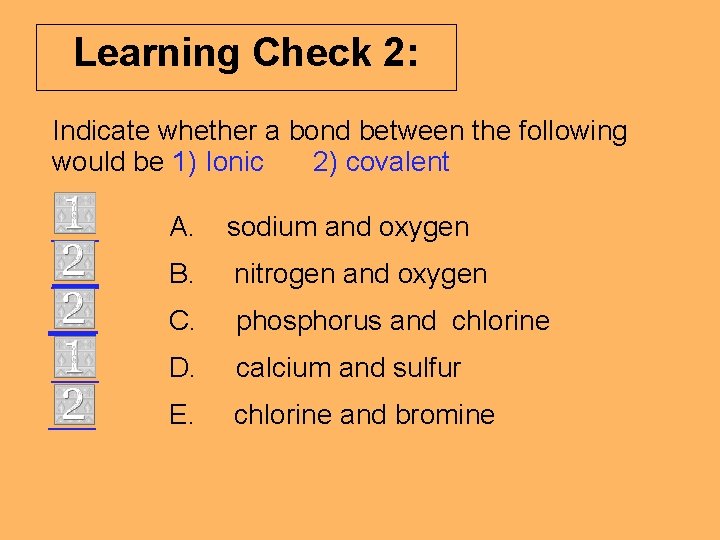
Learning Check 2: Indicate whether a bond between the following would be 1) Ionic 2) covalent ___ A. sodium and oxygen ___ B. nitrogen and oxygen ___ C. phosphorus and chlorine ___ D. calcium and sulfur ___ E. chlorine and bromine

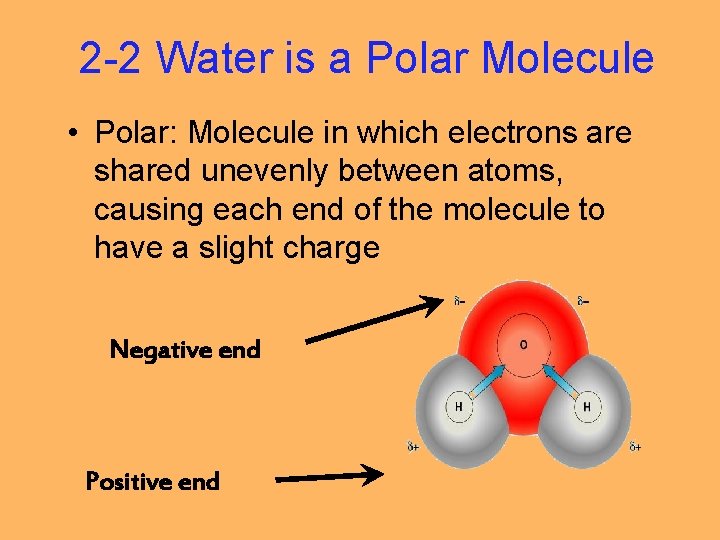
2 -2 Water is a Polar Molecule • Polar: Molecule in which electrons are shared unevenly between atoms, causing each end of the molecule to have a slight charge Negative end Positive end

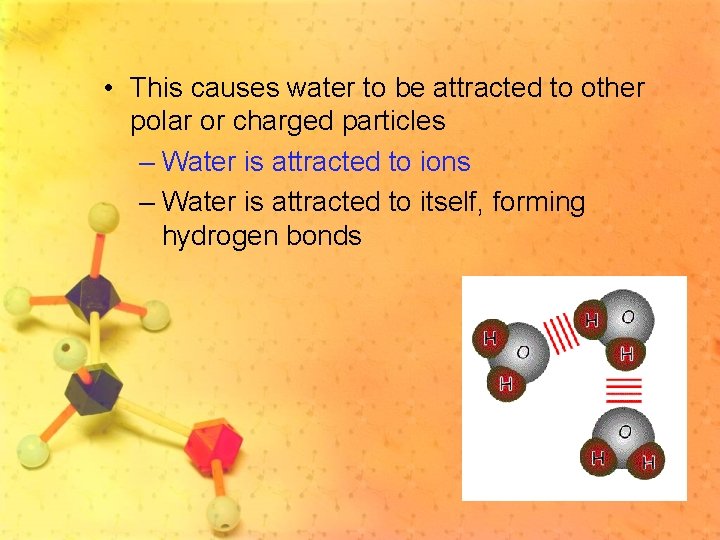
• This causes water to be attracted to other polar or charged particles – Water is attracted to ions – Water is attracted to itself, forming hydrogen bonds

Hydrogen Bonds In Water Are Responsible For: • Adhesion – Attraction between molecules of different substances – Graduated cylinder • Cohesion – Attraction between molecules of the same substance – Drops of water on a penny • Ex: Surface Tension • Jesus Lizard

Types of Chemical Substances • Compounds and Elements are called pure substances. • Most matter is neither of these.

Mixtures • Mixtures are combinations of substances held together by physical forces, not chemical bonds. • Each substance keeps its own properties

Mixtures may be either: §Solutions §Colloids §Suspensions

Solutions • Have small particles • Are transparent (not the same as colorless) • Do not separate • Water solutions are very common in biological systems – Examples: salt water, kool-aid, air, brass, vinegar

Colloids • Have medium size particles • Do not separate – Examples: fog, whipped cream, milk, cheese, mayonnaise

Suspensions • Have very large particles • Settle out (separates into layers) – Examples: blood platelets, muddy water, calamine lotion, oil & water, Italian salad dressing

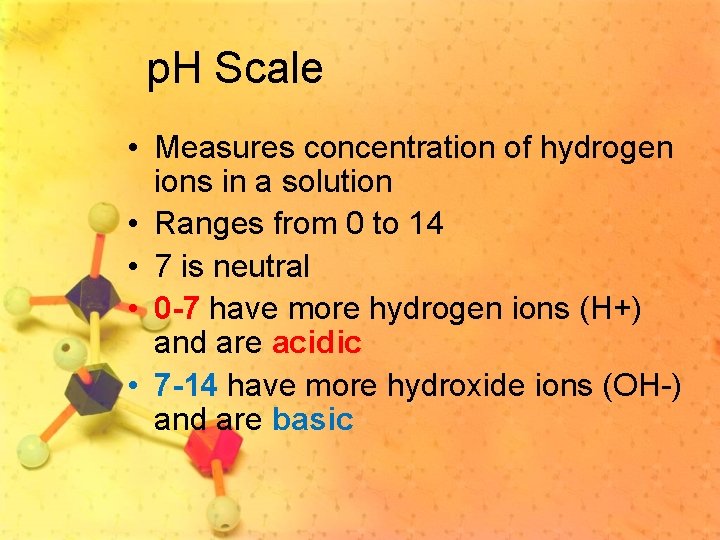
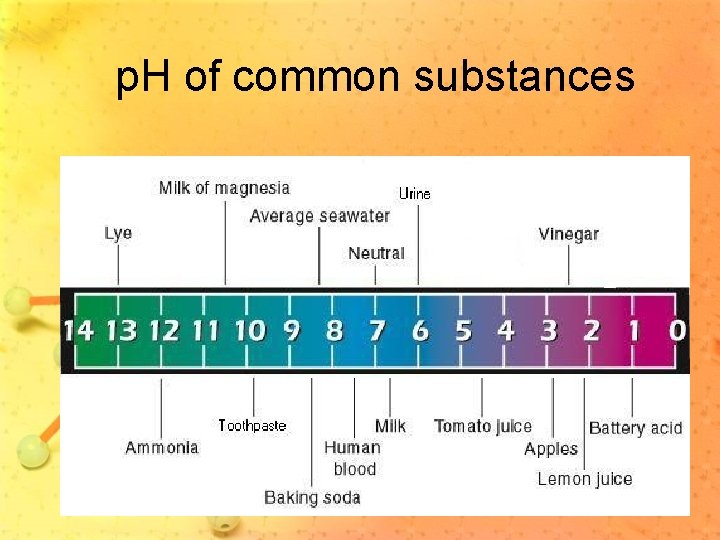
p. H Scale • Measures concentration of hydrogen ions in a solution • Ranges from 0 to 14 • 7 is neutral • 0 -7 have more hydrogen ions (H+) and are acidic • 7 -14 have more hydroxide ions (OH-) and are basic

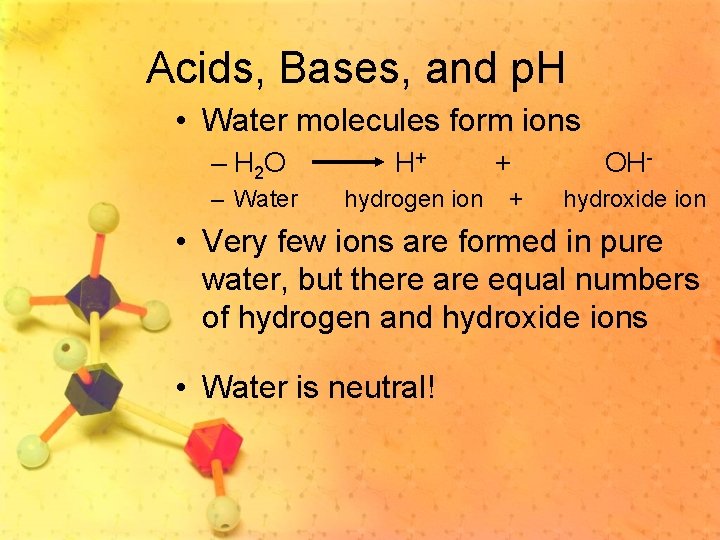
Acids, Bases, and p. H • Water molecules form ions – H 2 O H+ – Water hydrogen ion + + OHhydroxide ion • Very few ions are formed in pure water, but there are equal numbers of hydrogen and hydroxide ions • Water is neutral!

p. H of common substances

p. H and Homeostasis • Maintaining a p. H between 6. 5 and 7. 5 is important in cells • Dissolved compounds called buffers control p. H – Proteins – Phosphates – Hydrogen carbonate

Chemical Reactions • When one set of chemicals changes into another set of chemicals, a chemical reaction occurs • Bonds are either broken or formed (or both!)

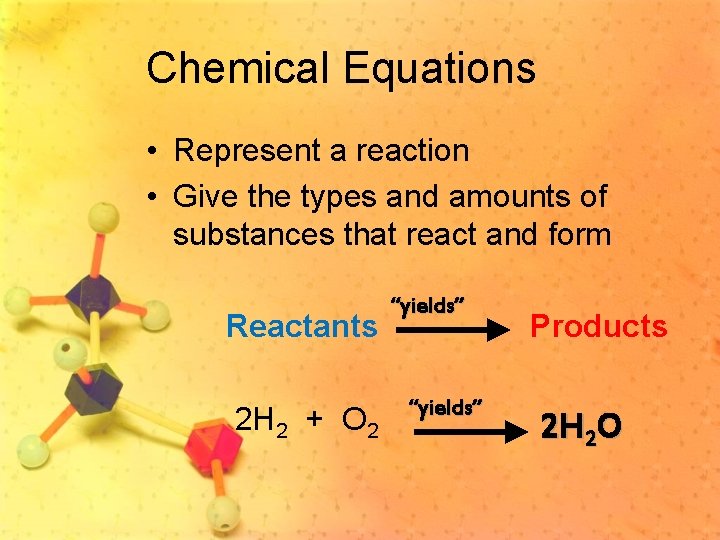
Chemical Equations • Represent a reaction • Give the types and amounts of substances that react and form Reactants 2 H 2 + O 2 “yields” Products 2 H 2 O

Evidence of a Chemical Reaction • Formation of a precipitate (a solid substance separated from a liquid) • Gas is evolved (seen by bubbles forming in a liquid) • Change in heat or light energy

Organic Compounds


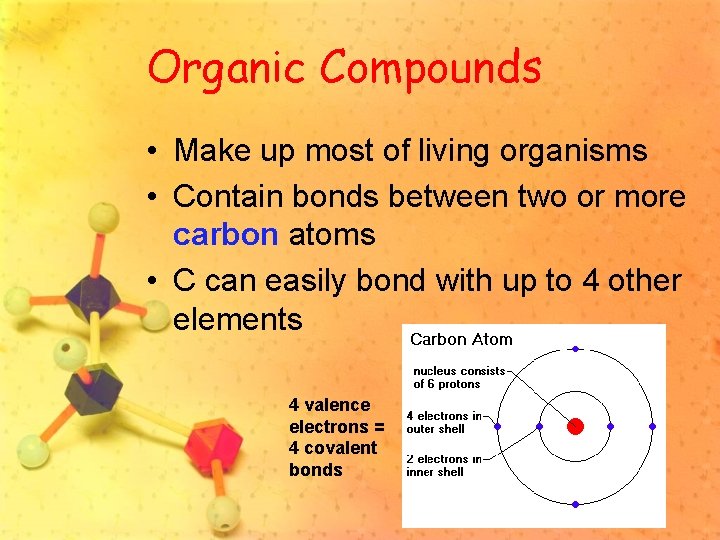
Organic Compounds • Make up most of living organisms • Contain bonds between two or more carbon atoms • C can easily bond with up to 4 other elements 4 valence electrons = 4 covalent bonds

Organic Compounds • Carbon atom is versatile, can be “backbone” of long chains or rings • Organic molecules can be extremely large and complex; these are called macromolecules

Organic Compounds • Four main types of organic macromolecules: Carbohydrates Lipids Proteins Nucleic Acids

Carbohydrates • Made of C, H, & O • Main energy source for living things • Breakdown of sugars supplies immediate energy for cell activities • Extra sugar is stored as complex carbs called starches

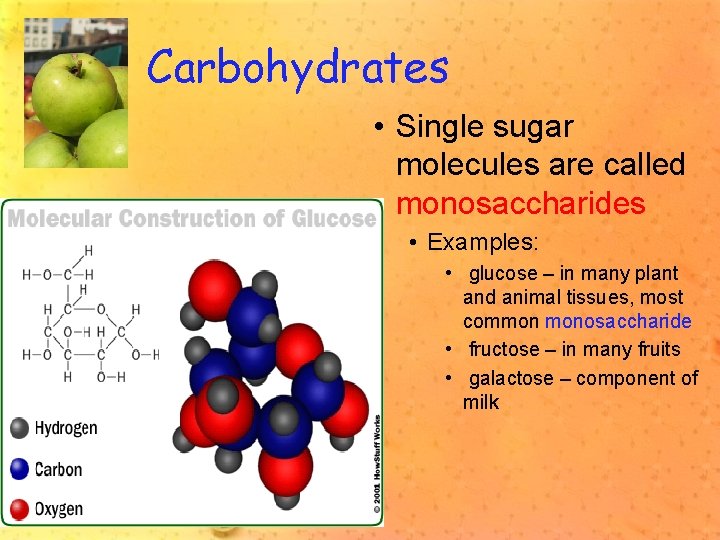
Carbohydrates • Single sugar molecules are called monosaccharides • Examples: • glucose – in many plant and animal tissues, most common monosaccharide • fructose – in many fruits • galactose – component of milk

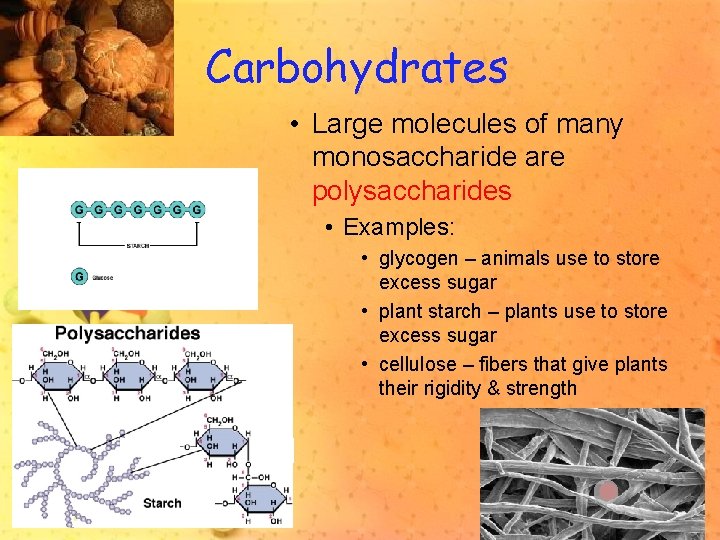
Carbohydrates • Large molecules of many monosaccharide are polysaccharides • Examples: • glycogen – animals use to store excess sugar • plant starch – plants use to store excess sugar • cellulose – fibers that give plants their rigidity & strength


Lipids • Store more energy than CHOs because the chains are longer • Ex: Fats, oils, waxes • Won’t dissolve in water

Lipids • Important parts of biological membranes and waterproof coverings • Steroids are lipids that act as chemical messengers

Lipids • Many lipids are made from a glycerol combined with fatty acids – If all carbons have single bonds, lipid is saturated – Ex: butter, lard, animal fat (usually solid at room temperature) – If any carbons have double or triple bonds, lipid is unsaturated – Ex: vegetable oil, fish oil, peanut oil room temperature) (usually liquid at


Proteins • Contain C, H, O, plus nitrogen • Formed from amino acids joined together • More than 20 amino acids can be joined in any order or number to make countless proteins (think of how many words can be made from 26 letters!)

Proteins • Chains are folded and twisted giving each protein a unique shape • Van der Waals forces and hydrogen bonds help maintain protein’s shape • Shape of protein is important to its function!

Proteins • Provide structure – Ex: Collagen- makes up your skin, muscles & bones • Aid chemical activities in your body – Ex: Enzymes- work to speed up rxns in your body • Transport substances into or out of cells • Help fight diseases


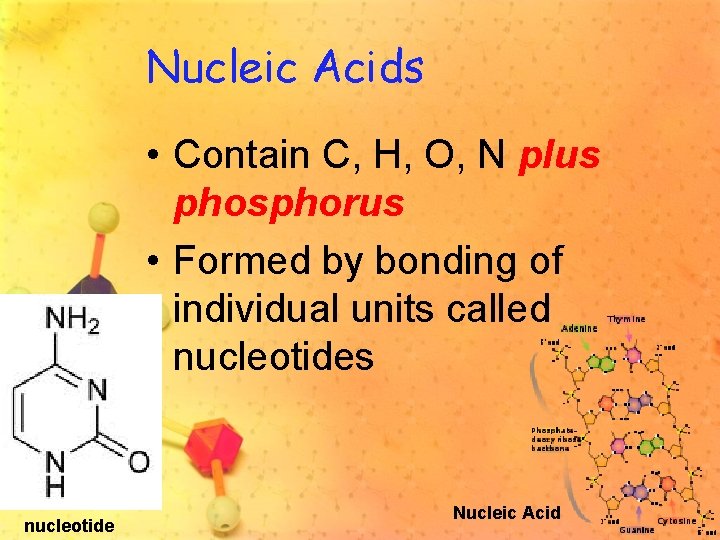
Nucleic Acids • Contain C, H, O, N plus phosphorus • Formed by bonding of individual units called nucleotides nucleotide Nucleic Acid

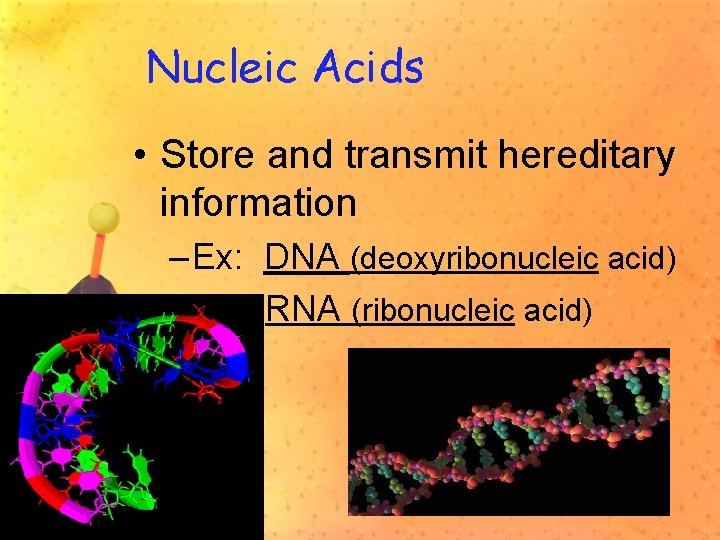
Nucleic Acids • Store and transmit hereditary information – Ex: DNA (deoxyribonucleic acid) RNA (ribonucleic acid)
