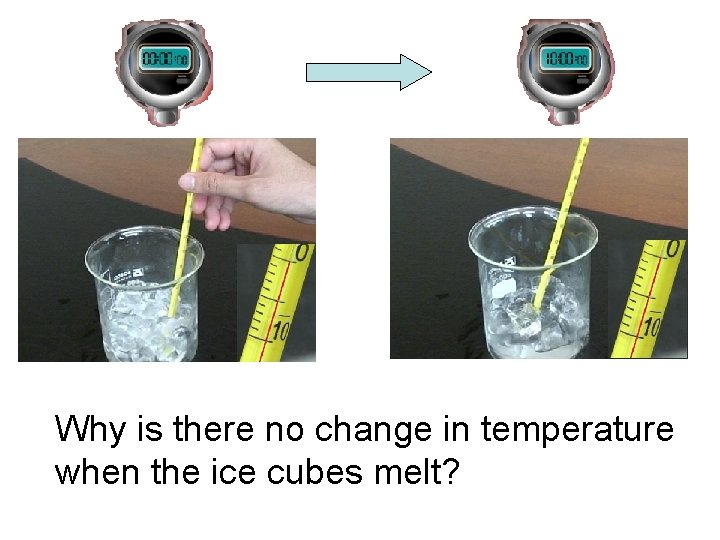
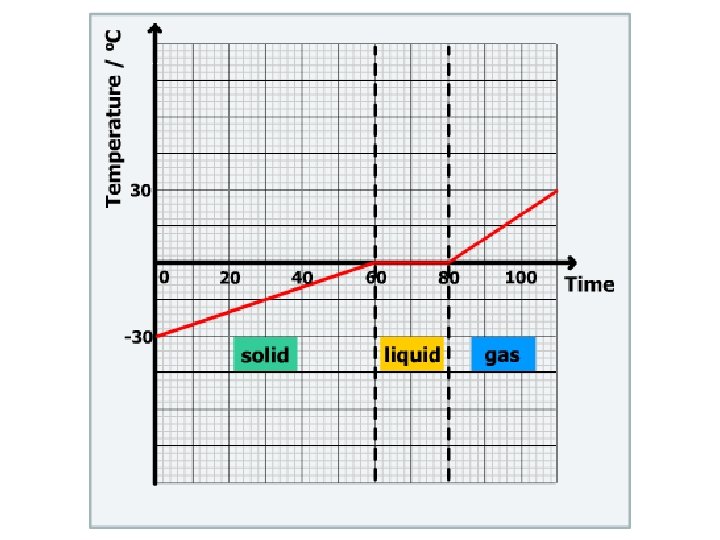
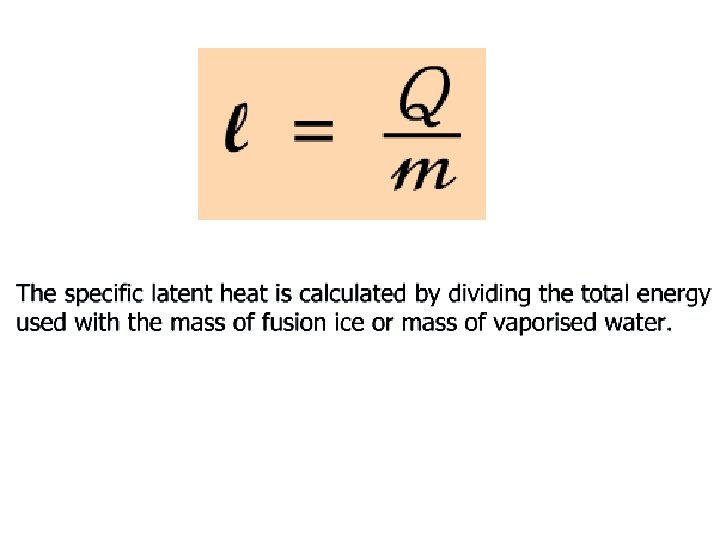Why is there no change in temperature when



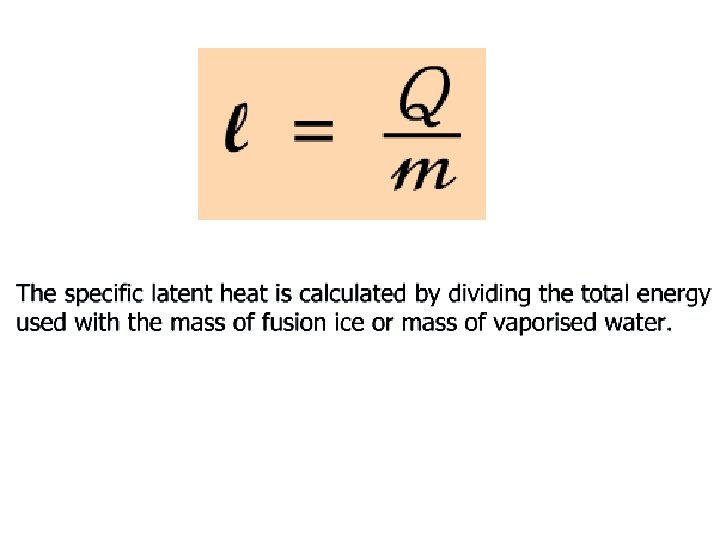



- Slides: 25



Why is there no change in temperature when the ice cubes melt?

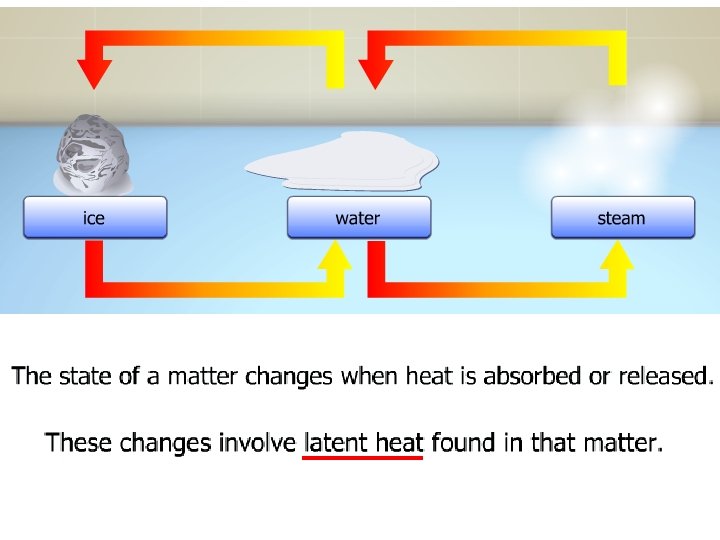
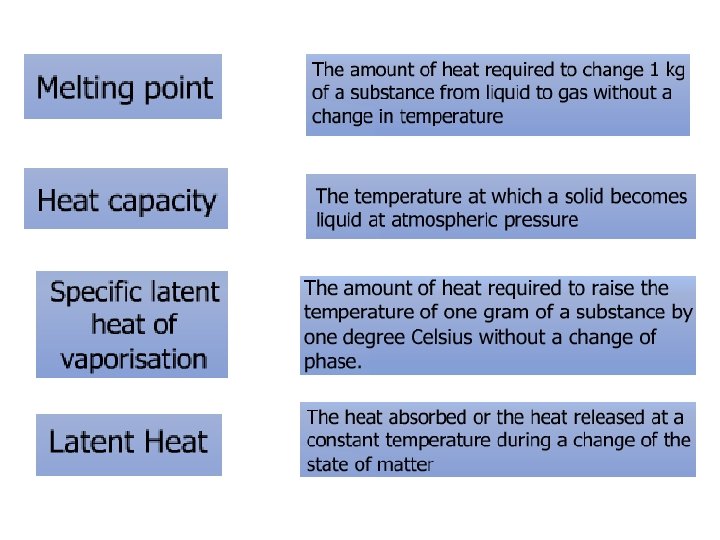
• The ice cubes have absorbed heat energy. • The energy melts the ice cube instead of increasing the temperature. • The heat absorbed or the heat released at a constant temperature during a change of the state of matter is known as latent heat.

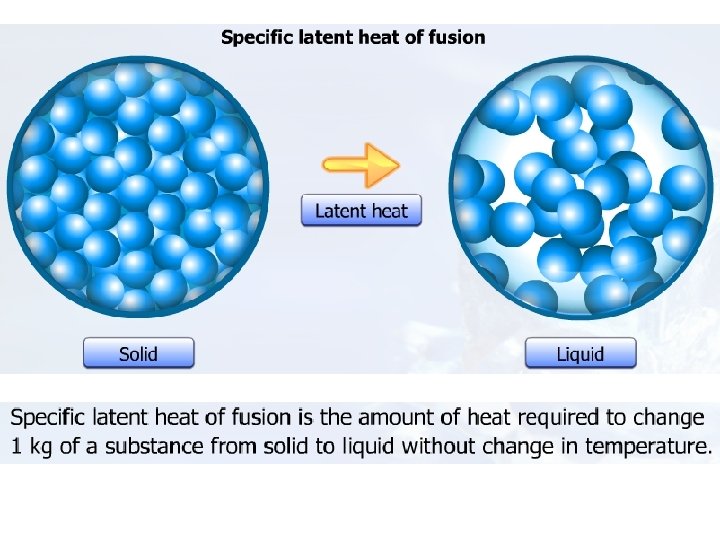
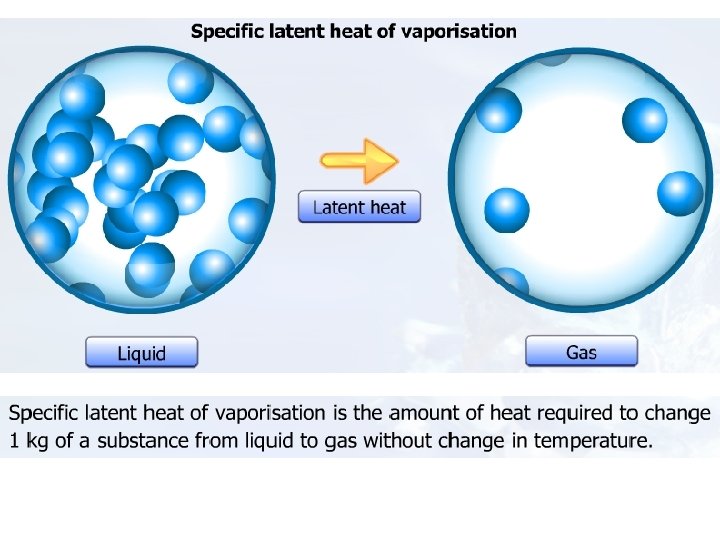
Specific latent heat

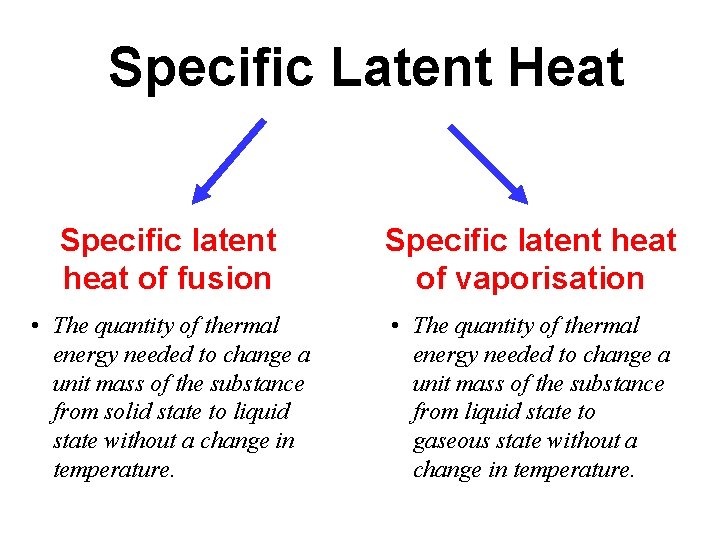
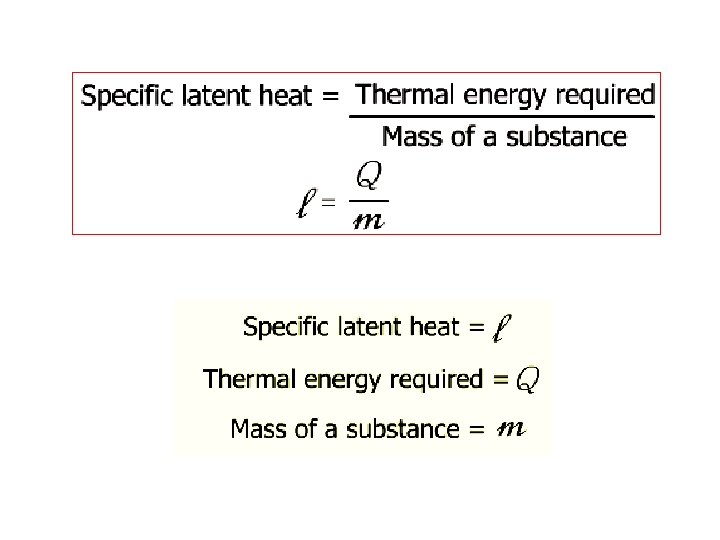
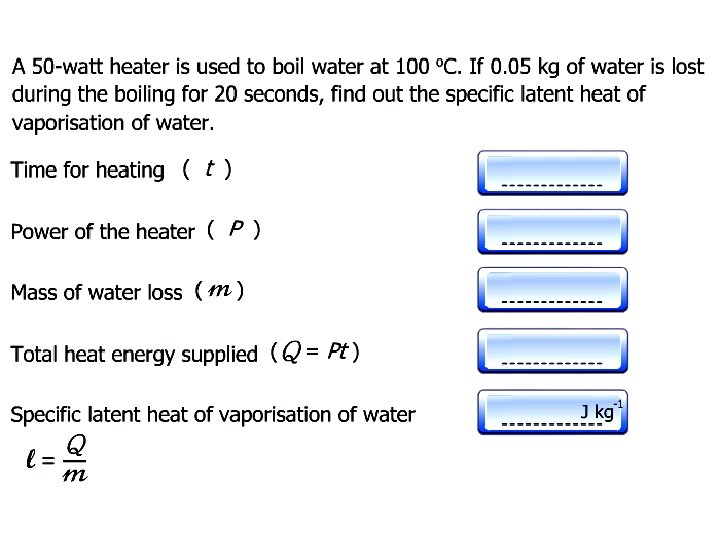
Specific Latent Heat Specific latent heat of fusion Specific latent heat of vaporisation • The quantity of thermal energy needed to change a unit mass of the substance from solid state to liquid state without a change in temperature. • The quantity of thermal energy needed to change a unit mass of the substance from liquid state to gaseous state without a change in temperature.


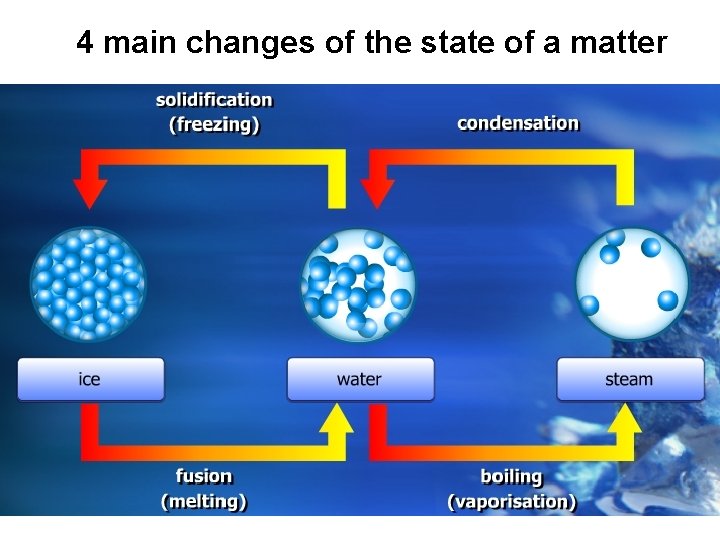
4 main changes of the state of a matter

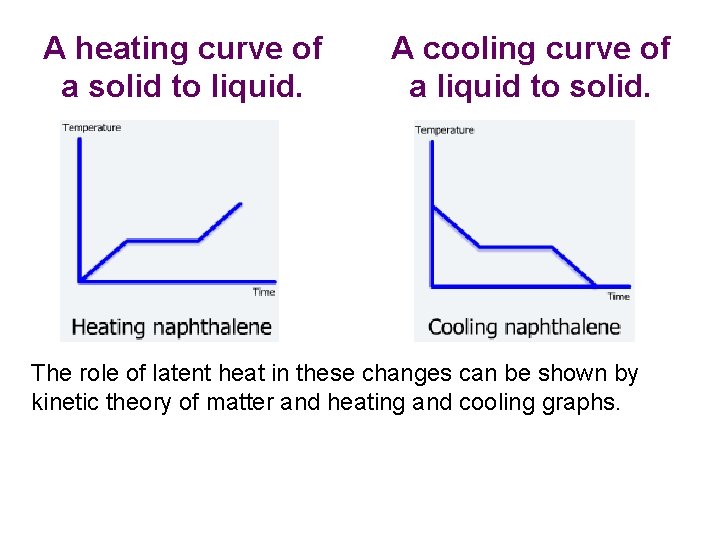
A heating curve of a solid to liquid. A cooling curve of a liquid to solid. The role of latent heat in these changes can be shown by kinetic theory of matter and heating and cooling graphs.

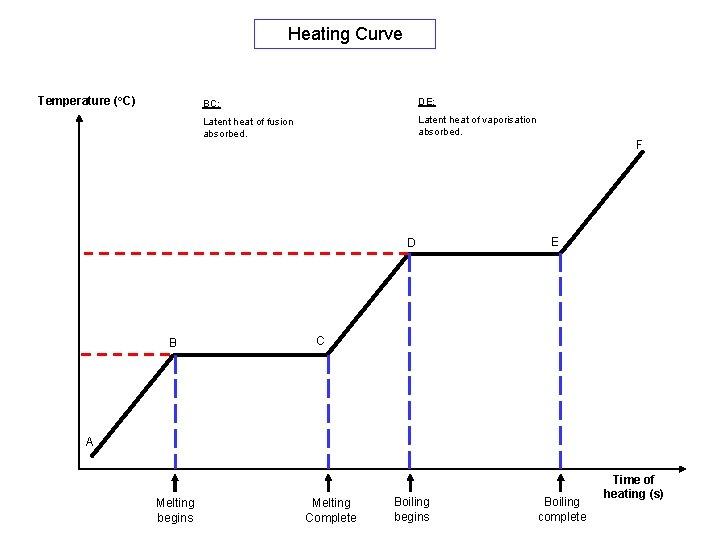
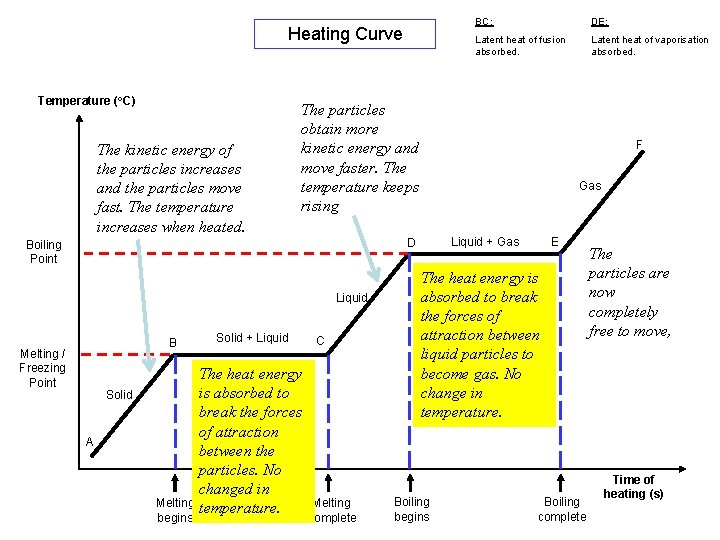
Heating Curve Temperature (o. C) BC: DE: Latent heat of fusion absorbed. Latent heat of vaporisation absorbed. F D B E C A Melting begins Melting Complete Boiling begins Boiling complete Time of heating (s)

Heating Curve Temperature (o. C) The kinetic energy of the particles increases and the particles move fast. The temperature increases when heated. Liquid Solid A Melting begins Latent heat of fusion absorbed. Latent heat of vaporisation absorbed. F Gas Liquid + Gas D Melting / Freezing Point DE: The particles obtain more kinetic energy and move faster. The temperature keeps rising Boiling Point B BC: Solid + Liquid The heat energy is absorbed to break the forces of attraction between the particles. No changed in temperature. C Melting Complete E The heat energy is absorbed to break the forces of attraction between liquid particles to become gas. No change in temperature. Boiling begins Boiling complete The particles are now completely free to move, Time of heating (s)

Cooling Curve Temperature (o. C) G H I J K L Time of cooling (s)

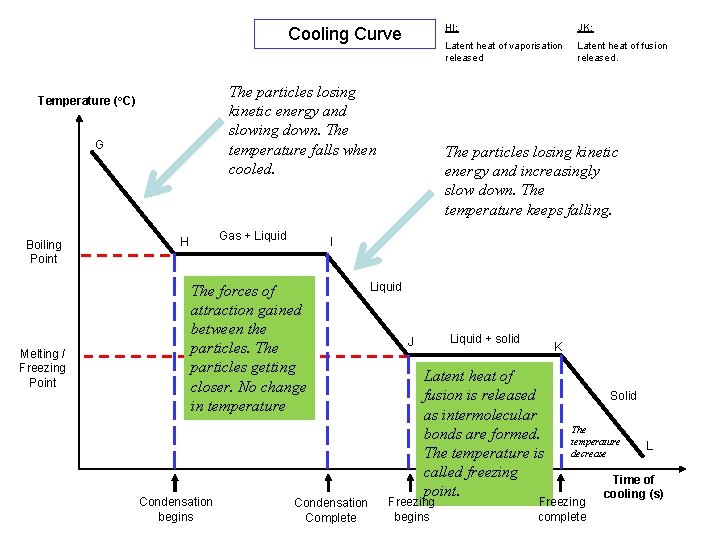
Cooling Curve The particles losing kinetic energy and slowing down. The temperature falls when cooled. Temperature (o. C) G Boiling Point Melting / Freezing Point Gas + Liquid H JK: Latent heat of vaporisation released Latent heat of fusion released. The particles losing kinetic energy and increasingly slow down. The temperature keeps falling. I The forces of attraction gained between the particles. The particles getting closer. No change in temperature Condensation begins HI: Condensation Complete Liquid + solid J K Latent heat of fusion is released as intermolecular bonds are formed. The temperature is called freezing point. Freezing begins Solid The temperature decrease Freezing complete L Time of cooling (s)



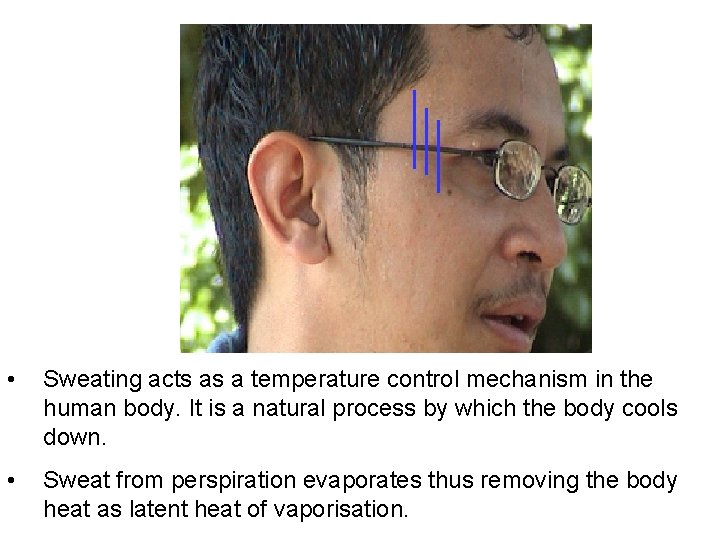
• Sweating acts as a temperature control mechanism in the human body. It is a natural process by which the body cools down. • Sweat from perspiration evaporates thus removing the body heat as latent heat of vaporisation.