Why is chirality important in drug development Biological

Why is chirality important in drug development? • Biological systems like that of human beings have exhibited chirality. • This is reflected by the existence of chirality of drug receptor areas and the requirement of chiral specificity on drugs. • It follows the lock-and-key hypothesis proposed by the famous scientist Emil Fischer.

One enantiomer of a drug is likely to interact much better than the other, or perhaps in a different way altogether, so the two enantiomers of chiral drugs often have quite different pharmacological effects. In the case of naproxen, the (S )-enantiomer is 28 times as effective as the (R ). Ibuprofen, on the other hand, is still marketed as a racemate because the two enantiomers have more or less the same painkilling effect.
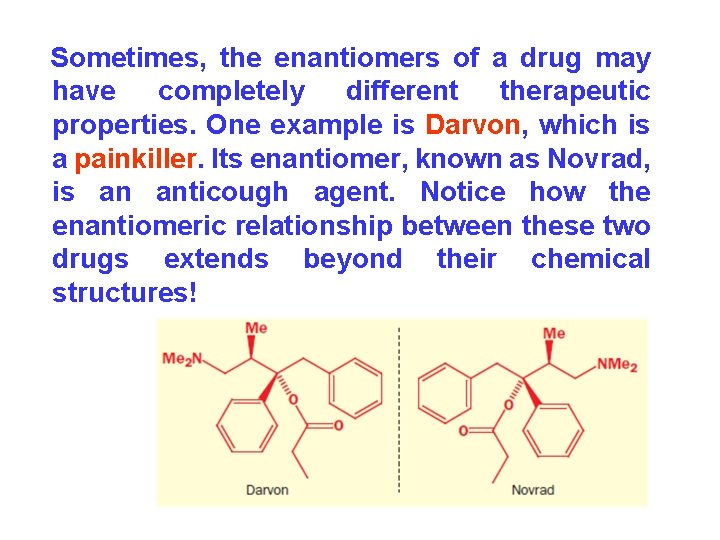
Sometimes, the enantiomers of a drug may have completely different therapeutic properties. One example is Darvon, which is a painkiller. Its enantiomer, known as Novrad, is an anticough agent. Notice how the enantiomeric relationship between these two drugs extends beyond their chemical structures!
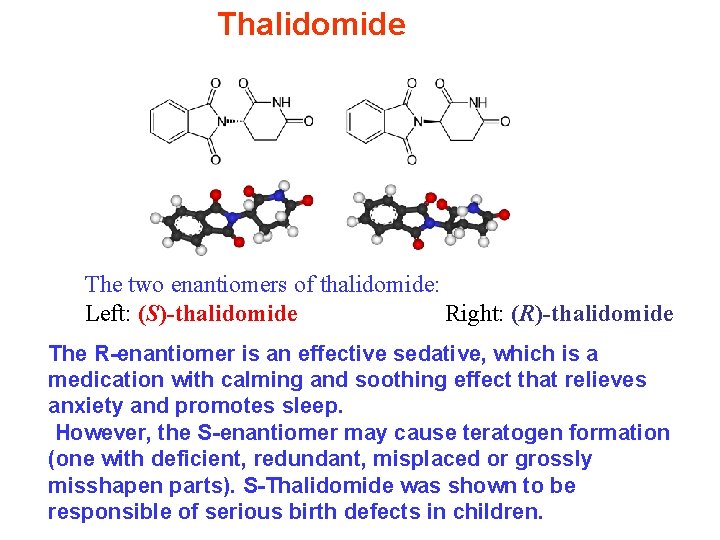
Thalidomide The two enantiomers of thalidomide: Left: (S)-thalidomide Right: (R)-thalidomide The R-enantiomer is an effective sedative, which is a medication with calming and soothing effect that relieves anxiety and promotes sleep. However, the S-enantiomer may cause teratogen formation (one with deficient, redundant, misplaced or grossly misshapen parts). S-Thalidomide was shown to be responsible of serious birth defects in children.

Stereochemistry and Drug Action ü Positional Isomers Same molecular formula, same functional groups, but different positions of functional groups.
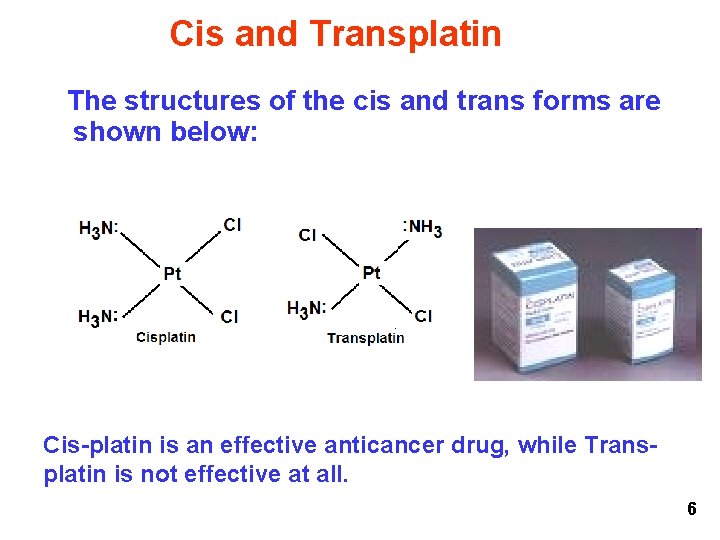
Cis and Transplatin The structures of the cis and trans forms are shown below: Cis-platin is an effective anticancer drug, while Transplatin is not effective at all. 6
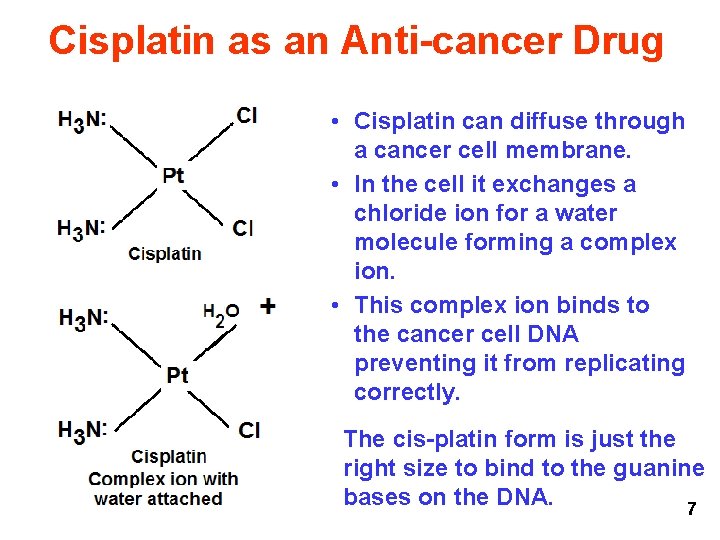
Cisplatin as an Anti-cancer Drug • Cisplatin can diffuse through a cancer cell membrane. • In the cell it exchanges a chloride ion for a water molecule forming a complex ion. • This complex ion binds to the cancer cell DNA preventing it from replicating correctly. The cis-platin form is just the right size to bind to the guanine bases on the DNA. 7

- Slides: 8