Why does Mentos make Cola explode o o










- Slides: 18

Why does Mentos make Cola “explode“? o o o Introduction The Phenomenon Different Explanations The Composition of Cola CO 2 in Cola Henry‘s Law Two States of Equilibrium CO 2 in Solution The Bubbles – Young-Laplace The Bubbles – Nucleation The Mentos Effect Coca Cola vs. Diet Coke vs. Cheap Cola Philipp Schüdel- Why does Mentos make Cola “explode”? 1

Introduction Philipp Schüdel- Why does Mentos make Cola “explode”? 2

The Phenomenon l White Mentos, thrown into a bottle of Cola, results in a violent eruption of the soda-drink l It is found, that this works best with Diet Coke but the principle works with any drink containing CO 2 l Why does that happen? l Why does it work best with Diet Coke? Philipp Schüdel- Why does Mentos make Cola “explode”? 3

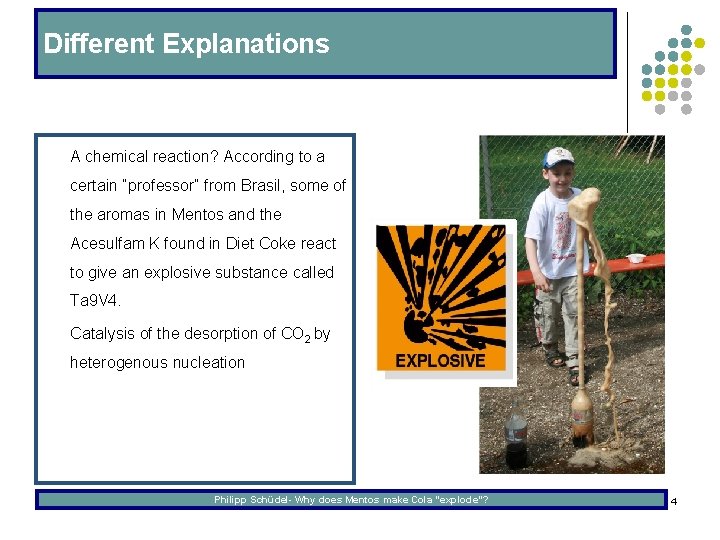
Different Explanations l A chemical reaction? According to a certain “professor“ from Brasil, some of the aromas in Mentos and the Acesulfam K found in Diet Coke react to give an explosive substance called Ta 9 V 4. l Catalysis of the desorption of CO 2 by heterogenous nucleation Philipp Schüdel- Why does Mentos make Cola “explode”? 4

The Composition of Cola l Main ingredients of normal Coca Cola: water, sugar, CO 2 (called carbonic acid), phosphorus acid, food dyes, other l Main ingredients of Diet Coke: water, CO 2 (called carbonic acid), phosphorus acid, citric acid, acesulfame K, aspartame, food dyes, other l => Most probable reason for violent eruption: CO 2 Philipp Schüdel- Why does Mentos make Cola “explode”? 5

CO 2 in Cola l CO 2 is dissolved in the Cola Mixture by applying a pressure of approximately 4 atmospheres l Questions: - What does dissolved CO 2 look like? - What happens when the pressure is reset to 1 atm? (opening of the bottle) l In approximation, Cola can be described as a solution of CO 2 in water. For such a system, Henry‘s law can be applied. Philipp Schüdel- Why does Mentos make Cola “explode”? 6

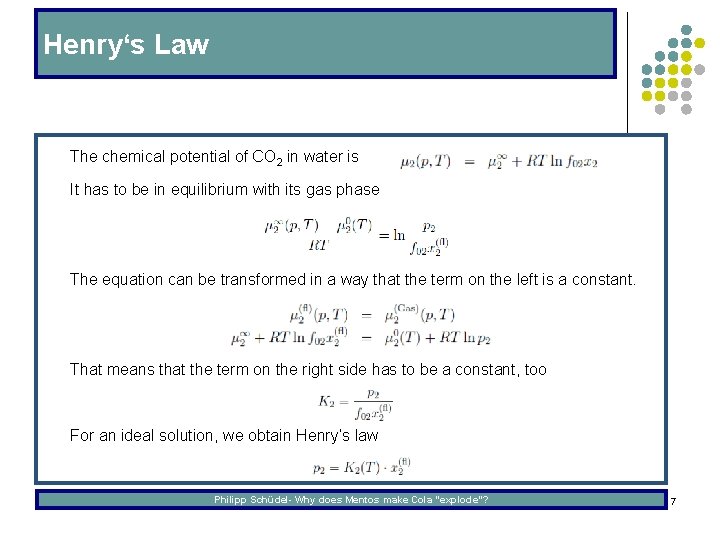
Henry‘s Law l The chemical potential of CO 2 in water is l It has to be in equilibrium with its gas phase l The equation can be transformed in a way that the term on the left is a constant. l That means that the term on the right side has to be a constant, too l For an ideal solution, we obtain Henry‘s law Philipp Schüdel- Why does Mentos make Cola “explode”? 7

The Phenomenon l Another interesting application of Henry‘s law: The so called “Martini Effect“: Divers can suffer from a sort of drunkness in deep waters. This is due to the higher solubility of N 2 in blood at higher pressure (Henry) l An empirical law connects the depth of the diver to the amount of Martinis he would have to drink in order to get the same effect. Every ten meters corresponds to one Martini where the feeling of being drunk starts at 30 meters. l Because a Martini is not a very common unit, a recalculation has been done to form the “Beer Effect“ according to which every 17 meters is like drinking half a litre of beer. Philipp Schüdel- Why does Mentos make Cola “explode”? 8

Two States of Equilibrium l Henry‘s law can also be written this way: c(CO 2)= KH*p(CO 2) with KH= 0. 034 atm*l*mol-1 at 25°C l Sealed bottle of Cola: p(CO 2)= 4 atm l => Opened bottle: p(CO 2)= 2. 96*10 -4 atm => l c(CO 2)= 0. 136 mol*l-1 c(CO 2)= 0, 010 mmol*l-1 How does Mentos help in getting from one state of equilibrium to the other? l What does CO 2 dissolved in water look like? Philipp Schüdel- Why does Mentos make Cola “explode”? 9

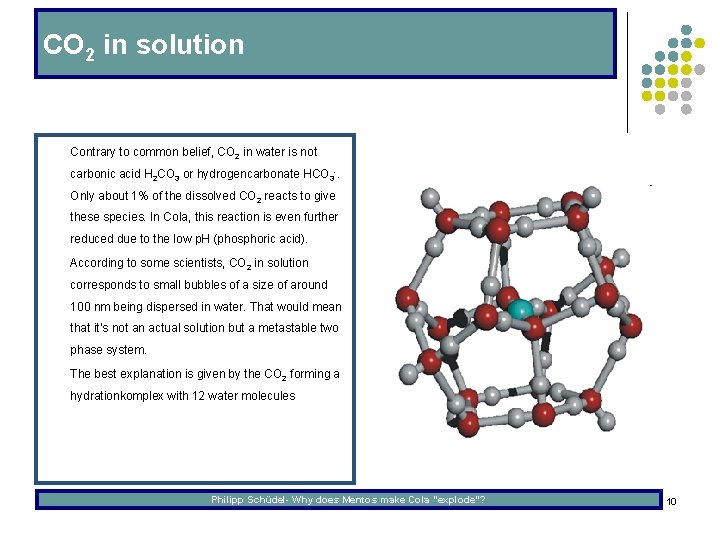
CO 2 in solution l Contrary to common belief, CO 2 in water is not carbonic acid H 2 CO 3 or hydrogencarbonate HCO 3 -. Only about 1% of the dissolved CO 2 reacts to give these species. In Cola, this reaction is even further reduced due to the low p. H (phosphoric acid). l According to some scientists, CO 2 in solution corresponds to small bubbles of a size of around 100 nm being dispersed in water. That would mean that it‘s not an actual solution but a metastable two phase system. l The best explanation is given by the CO 2 forming a hydrationkomplex with 12 water molecules Philipp Schüdel- Why does Mentos make Cola “explode”? 10

The Bubbles - Young-Laplace l How does the system get to equilibrium? l Bubbles formed in the solution are only stable above a certain diameter due to the surface tension and the pressure difference between the inside and the outside of a bubble. l The free energy of the surface is G= 4 pr 2 g where g is the surface tension l A change of the radius of the bubble results in a change of the free energy: DG= 8 pgrdr l This is compensated by the pressure-volume-work Dp*d. V= Dp*4 pr 2 dr l In equilibrium, those two terms are the same and we obtain the Young-Laplace-Equation: Dp= 2 g/r l One can see that the difference in pressure and therefore the instability of the bubble is smaller for small surface tensions and big bubble diameters. Philipp Schüdel- Why does Mentos make Cola “explode”? 11

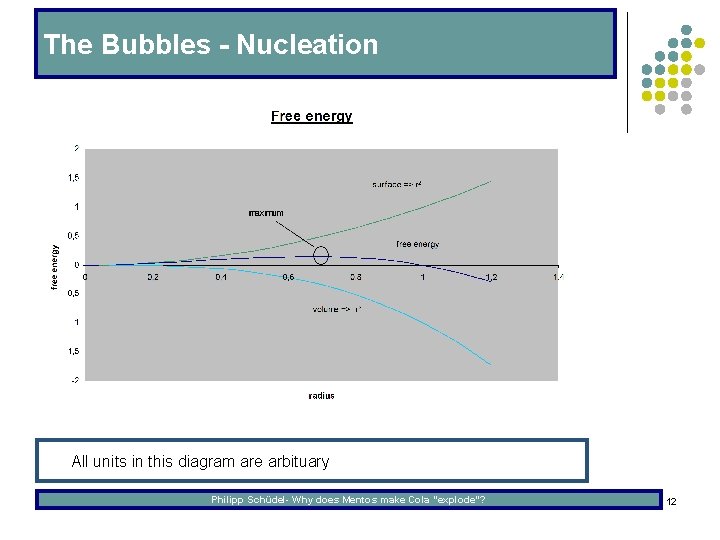
The Bubbles - Nucleation l All units in this diagram are arbituary Philipp Schüdel- Why does Mentos make Cola “explode”? 12

The Bubbles - Nucleation l In a homogeneous solution, the bubbles can only surpass the critical diameter through pressure fluctuations. This is called homogeneous nucleation. l Another example of homogenous nucleation are hand warming pads, where an overcritical liquid is crystallized by inducing nucleation through a charge emitted by a small metal plate. l The process of nucleation can also be catalyzed, which is called heterogeneous nucleation. A good example is rice which is put into Weizen. The CO 2 in the beer can form bubbles on the rice-surface which grow and make the rice “dance“. Philipp Schüdel- Why does Mentos make Cola “explode”? 13

The Bubbles - Nucleation l The formation of raindrops is also a nucleation process. Rain can only occur if small particles like dust, pollen etc. are present which is why it never rains in the Antarctic. l Furthermore, in regions with high particle concentration, like big cities, there are too many nucleation sites. Because of that, the raindrops can never grow enough in order to be able to fall. Philipp Schüdel- Why does Mentos make Cola “explode”? 14

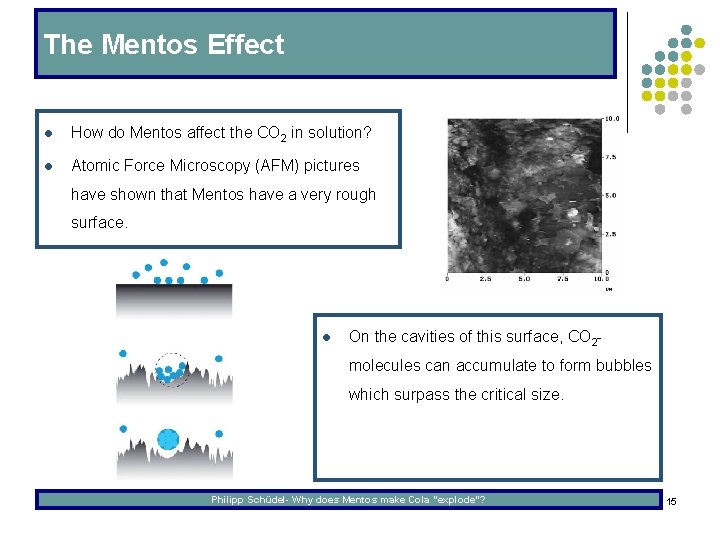
The Mentos Effect l How do Mentos affect the CO 2 in solution? l Atomic Force Microscopy (AFM) pictures have shown that Mentos have a very rough surface. l On the cavities of this surface, CO 2 molecules can accumulate to form bubbles which surpass the critical size. Philipp Schüdel- Why does Mentos make Cola “explode”? 15

Coca Cola vs. Diet Coke vs. Cheap Cola l Why does the Mentos-Cola experiment work best with Diet Coke? => Higher CO 2 concentration? l According to the Young-Laplace-equation ( Dp= 2 g/r ) a higher surface tension means that for a given bubble size, the pressure difference between the inside and the outside of the bubble is bigger than for a lower surface tension. That means that the bubbles in a system with high surface tension must be bigger in oder to be stable. l Surface tension measurements of normal Coca Cola, Diet Coke and cheap Cola showed that there is almost no difference. l The reason for a higher CO 2 concentration could also be a higher pressure applied in the production process, but an actual measurement of the amount of dissolved CO 2 showed no difference between the sodas. Philipp Schüdel- Why does Mentos make Cola “explode”? 16

Coca Cola vs. Diet Coke vs. Cheap Cola l In conclusion, the real reason why the experiment works best with Diet Coke has yet to be found. The problem seems to be a bit more complicated than expected and there is no valid explanation found so far. Philipp Schüdel- Why does Mentos make Cola “explode”? 17

Thank you for your attention and have a nice day! Philipp Schüdel- Why does Mentos make Cola “explode”? 18