WHY do properties change at the nanoscale An










- Slides: 26

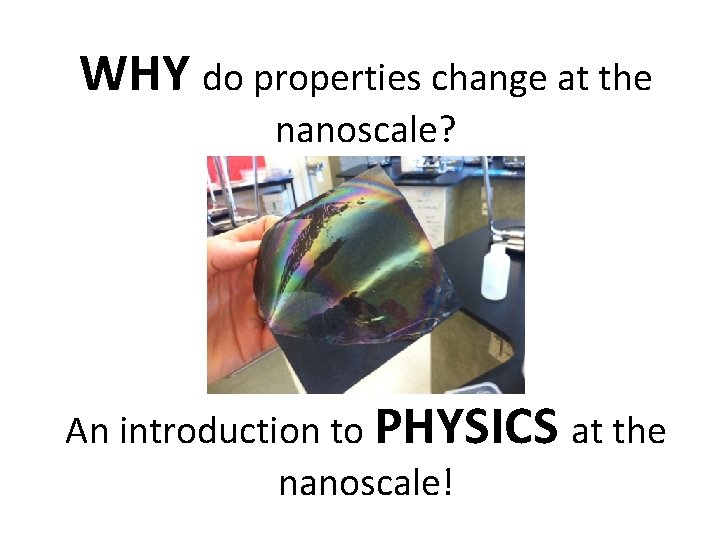
WHY do properties change at the nanoscale? An introduction to PHYSICS at the nanoscale!


Tiny Teacup Activity

Forces that win at the macroscale aren’t as important at the nanoscale.

When you tip a normal-sized teacup, why does the water pour out? Gravity! When you tip a tiny teacup, why doesn’t the water pour out? Properties of water like adhesion, cohesion, polarity & surface tension “defeat” gravity in the miniature version.

Styrofoam ball Throw!

When you throw a ball there are competing forces. Drag forces are proportional to surface area while inertia, which is proportional to volume, resists changes in velocity. How quickly an object slows down (its deceleration) is given simply by the ratio of the drag force to the inertia (mass). Deceleration = Drag force/Inertia = (C x Surface Area x Velocity)/(Density x Volume) C is a coefficient that depends on the fluid the object is moving through (so it would be the same for both styrofoam balls), we’re assuming the initial velocity & density are the same for both also. Styrofoam has the correct density where we can observe a crossover of the dominance of inertia (for the larger ball) to the dominance of viscous drag (air resistance) for the smaller ball.

The four important changes at the nanoscale… • Gravitational forces become negligible & electromagnetic forces dominate (+ & charges) • Quantum mechanics is used to describe motion rather than classical mechanics • Surface area to volume ratio increases • Random molecular motion becomes more important

BACKGROUND…

The Original Physics • Classical physics • Largely developed by Isaac Newton (late 1600 s) • Action = reaction • Still relevant to our world today Doesn’t explain observations of atoms, molecules, subatomic particles….

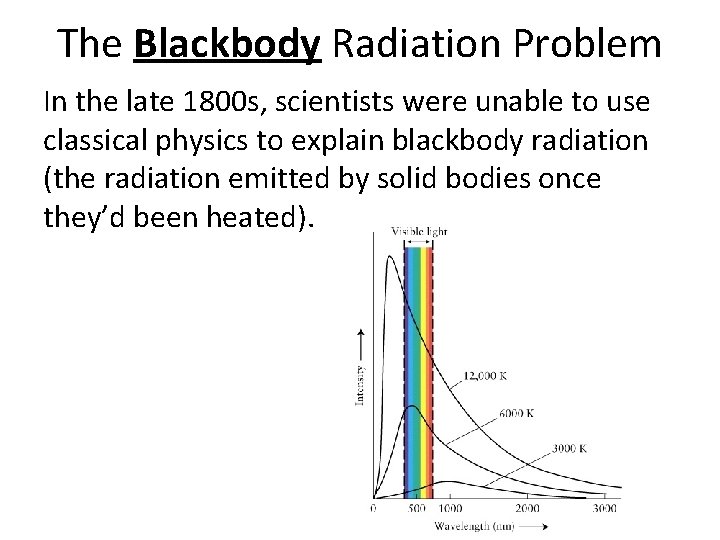
The Blackbody Radiation Problem In the late 1800 s, scientists were unable to use classical physics to explain blackbody radiation (the radiation emitted by solid bodies once they’d been heated).

The scientist who started to redefine physics… Max Planck was able to explain blackbody radiation with his famous equation: E = hf Energy is discharged in small packets called quanta. (Before this, physicists believed energy could be discharged in any amount, it was continuous).

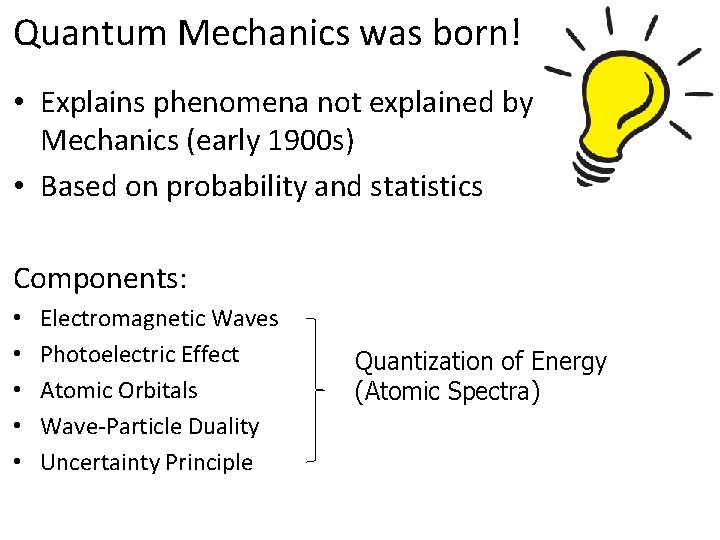
Quantum Mechanics was born! • Explains phenomena not explained by Classical Mechanics (early 1900 s) • Based on probability and statistics Components: • • • Electromagnetic Waves Photoelectric Effect Atomic Orbitals Wave-Particle Duality Uncertainty Principle Quantization of Energy (Atomic Spectra)

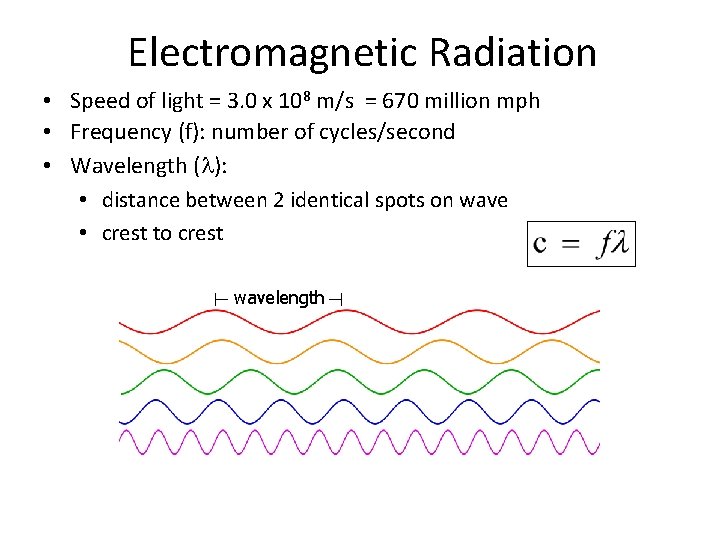
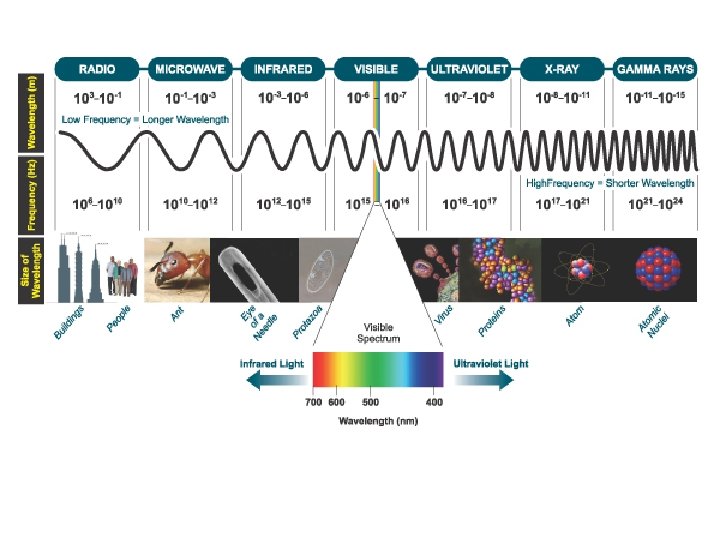
Electromagnetic Radiation • Speed of light = 3. 0 x 108 m/s = 670 million mph • Frequency (f): number of cycles/second • Wavelength ( ): • distance between 2 identical spots on wave • crest to crest wavelength


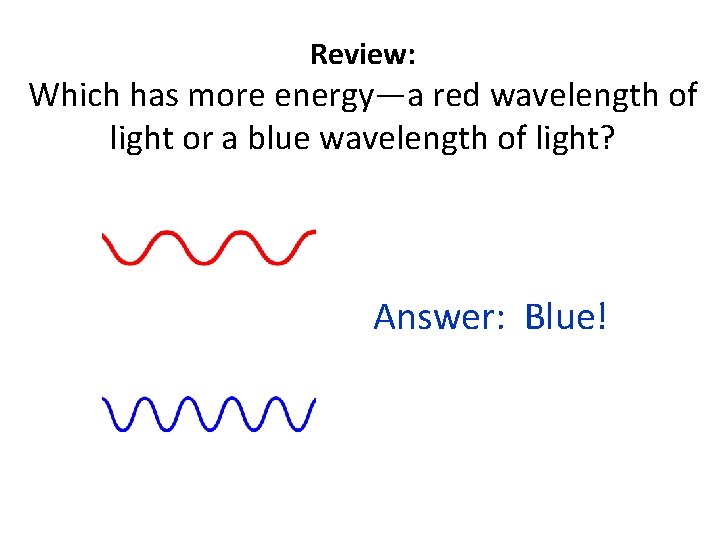
Review: Which has more energy—a red wavelength of light or a blue wavelength of light? Answer: Blue!

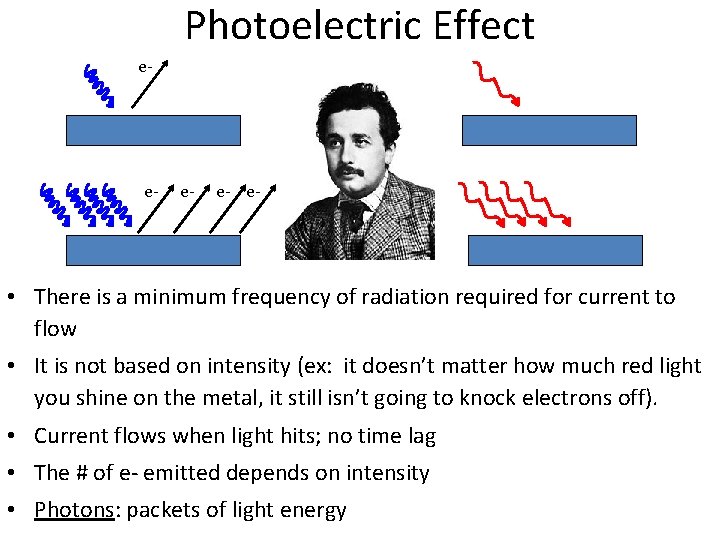
Photoelectric Effect e- e- e- • There is a minimum frequency of radiation required for current to flow • It is not based on intensity (ex: it doesn’t matter how much red light you shine on the metal, it still isn’t going to knock electrons off). • Current flows when light hits; no time lag • The # of e- emitted depends on intensity • Photons: packets of light energy

Photoelectric Effect Applications… • Digital cameras • Night vision • Solar cells

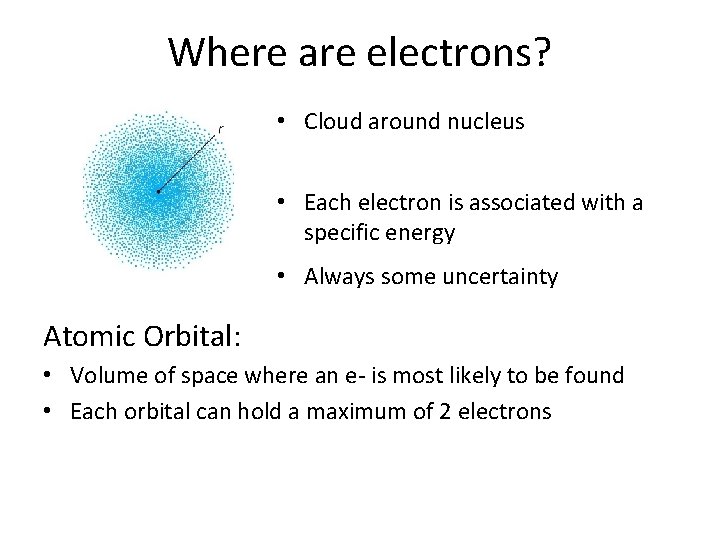
Where are electrons? • Cloud around nucleus • Each electron is associated with a specific energy • Always some uncertainty Atomic Orbital: • Volume of space where an e- is most likely to be found • Each orbital can hold a maximum of 2 electrons

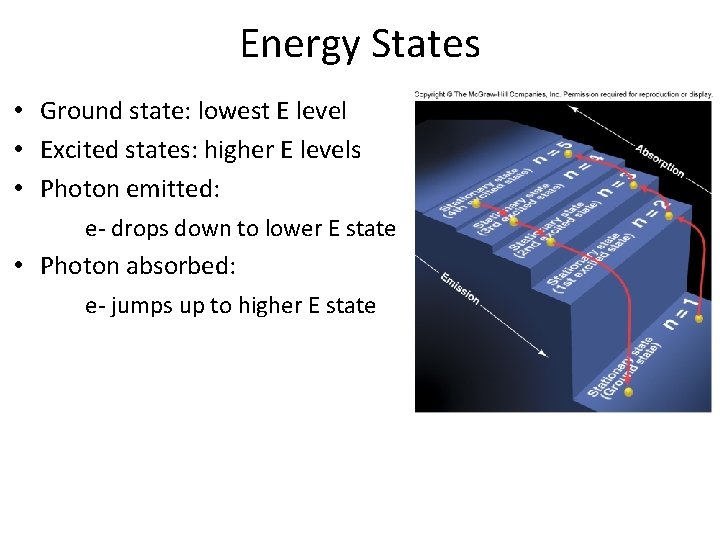
Energy States • Ground state: lowest E level • Excited states: higher E levels • Photon emitted: e- drops down to lower E state • Photon absorbed: e- jumps up to higher E state

Atomic Spectra 1. Electrons of gas atoms jump up energy levels (are “excited”) when you put energy in 2. Excited electrons drop down to the “ground” state The amount of energy lost is equal to the energy of light emitted

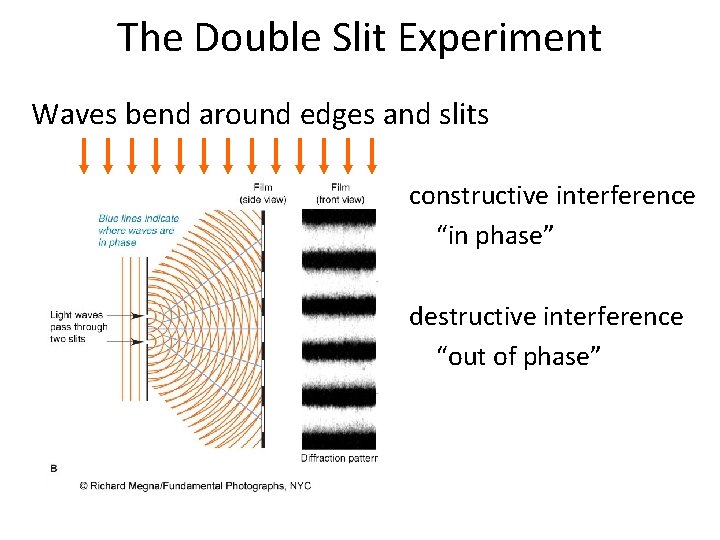
The Double Slit Experiment Waves bend around edges and slits constructive interference “in phase” destructive interference “out of phase”

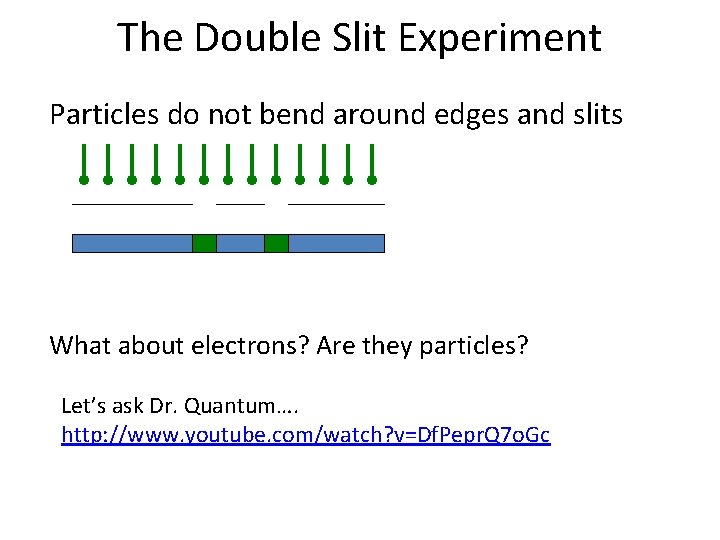
The Double Slit Experiment Particles do not bend around edges and slits What about electrons? Are they particles? Let’s ask Dr. Quantum…. http: //www. youtube. com/watch? v=Df. Pepr. Q 7 o. Gc

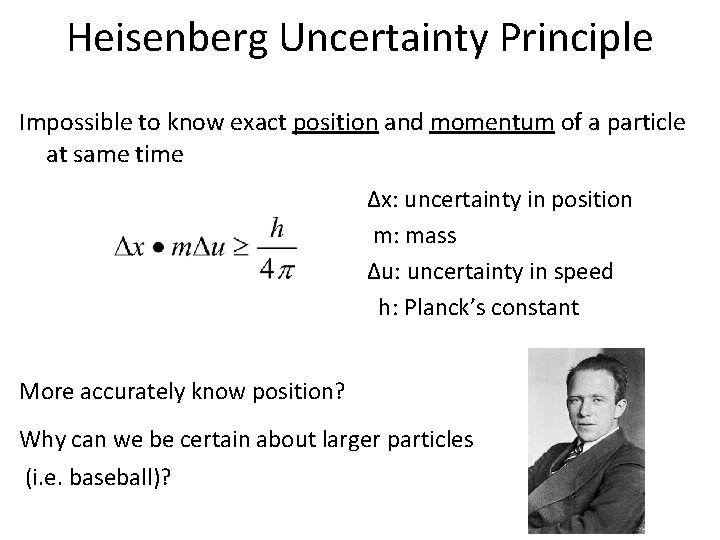
Heisenberg Uncertainty Principle Impossible to know exact position and momentum of a particle at same time ∆x: uncertainty in position m: mass ∆u: uncertainty in speed h: Planck’s constant More accurately know position? Why can we be certain about larger particles (i. e. baseball)?

Overview • Electrons in atoms are found in orbitals. • Each orbital has an energy level and sublevel (shape) • The closest energy level to the nucleus is the ground state; higher levels are excited states • Electrons can act like particles or waves (double-slit expt) • Radiation can act like energy or waves (photoelectric effect; photons are packets of light energy) • Atoms emit or absorb photons when their electrons change energy levels • The Uncertainty Principle states that the more you know an object’s position, the less you know its momentum (and visa versa).

Summary The laws of physics that are most important to a particular system depend on the size of that system. At the nanoscale, almost all interactions are mediated by surface effects. So forces between objects are often proportional to their surface area. This is why surface-related forces that bond molecules and nanoobjects together—such as chemical and intermolecular bonds—are so much more important than gravity! The surface area to volume is dramatically higher—up to a billion times higher—for nano-objects than for human-scale objects (so surface-related effects dominate).