Why are we studying water in Biology How

Why are we studying water in Biology? How does the habitat of a polar bear demonstrate the properties of water? AP Biology
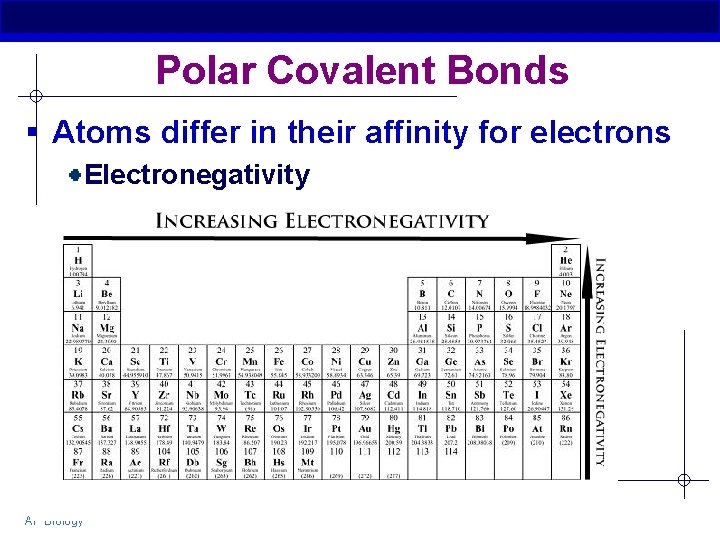
Polar Covalent Bonds § Atoms differ in their affinity for electrons Electronegativity AP Biology
Nonpolar vs Polar Covalent Bonds § Nonpolar Covalent Bonds Affinity for electrons is the same between identical atoms Electrons are shared equally & bond is nonpolar § Polar Covalent Bonds For atoms that differ greatly in electronegativity, electrons are not shared equally. Shared electrons will likely be closer to the atom with greater electronegativity Molecule is still electrically neutral but regions of partial (δ) negative & positive charges exist AP Biology
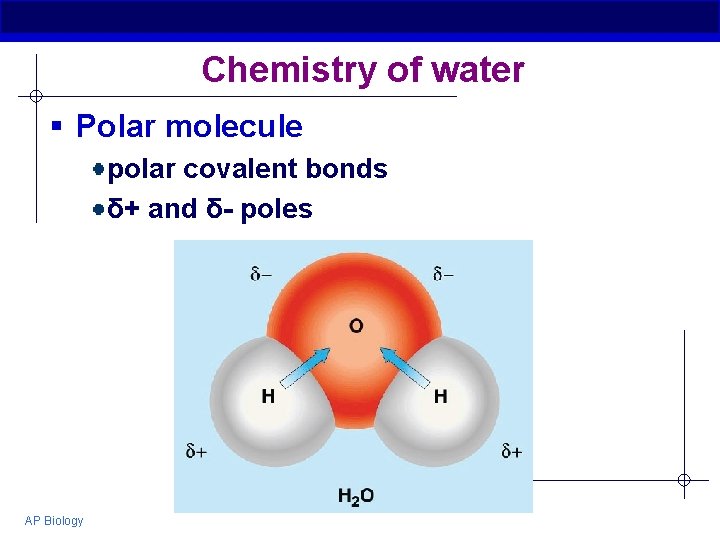
Chemistry of water § Polar molecule polar covalent bonds δ+ and δ- poles AP Biology
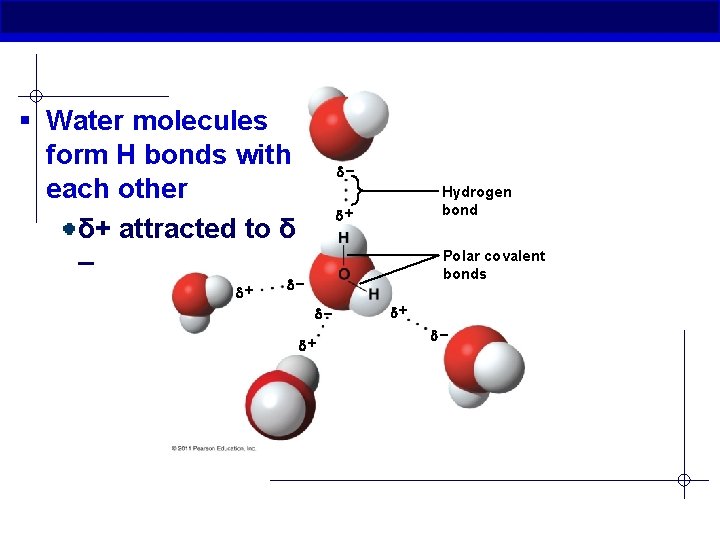
§ Water molecules form H bonds with each other δ+ attracted to δ – + Hydrogen bond + Polar covalent bonds + +

Properties of Water § Properties exist because of the polarity of water Cohesion Adhesion High Specific Heat High Heat of Vaporization Lower Density of Ice Solubility AP Biology
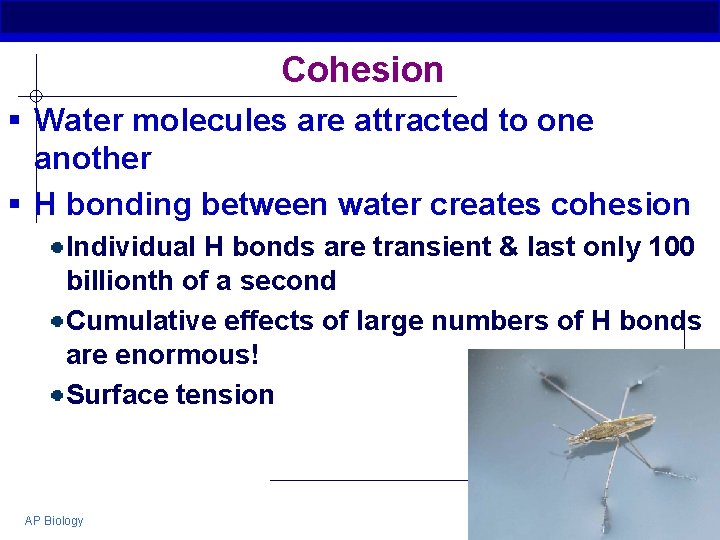
Cohesion § Water molecules are attracted to one another § H bonding between water creates cohesion Individual H bonds are transient & last only 100 billionth of a second Cumulative effects of large numbers of H bonds are enormous! Surface tension AP Biology
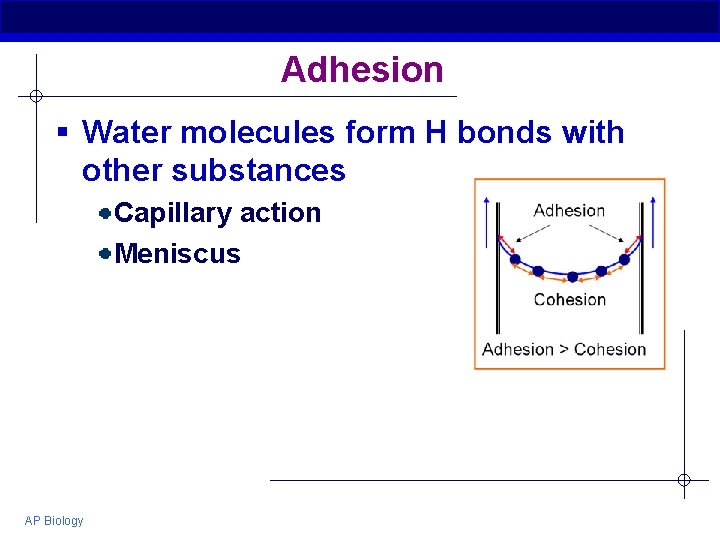
Adhesion § Water molecules form H bonds with other substances Capillary action Meniscus AP Biology

How does water get to top of tree? § Transpiration (cohesion and adhesion) AP Biology

Water is the solvent of life § Water is a good solvent due to its polarity § Polar water molecules surrounds + and – ions § Solvents dissolve solutes creating aqueous solutions AP Biology
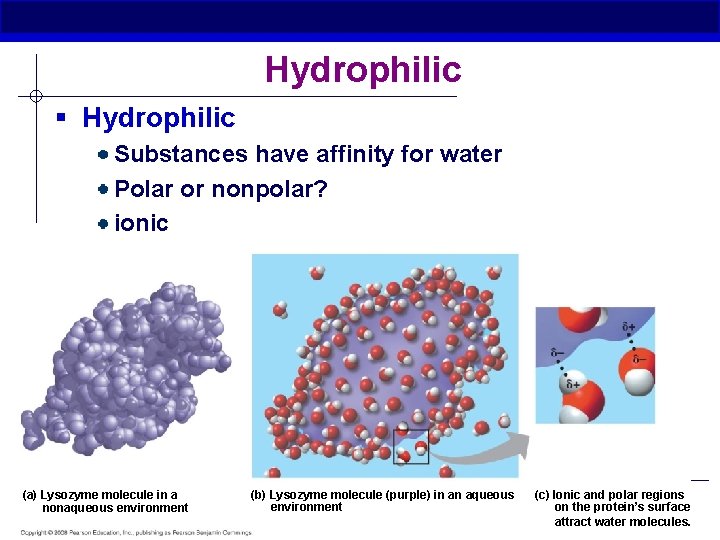
Hydrophilic § Hydrophilic Substances have affinity for water Polar or nonpolar? ionic (a) Lysozyme molecule in a nonaqueous environment AP Biology (b) Lysozyme molecule (purple) in an aqueous environment (c) Ionic and polar regions on the protein’s surface attract water molecules.

Hydrophobic § Substances do not have affinity for water § Polar or nonpolar? § Non-ionic AP Biology
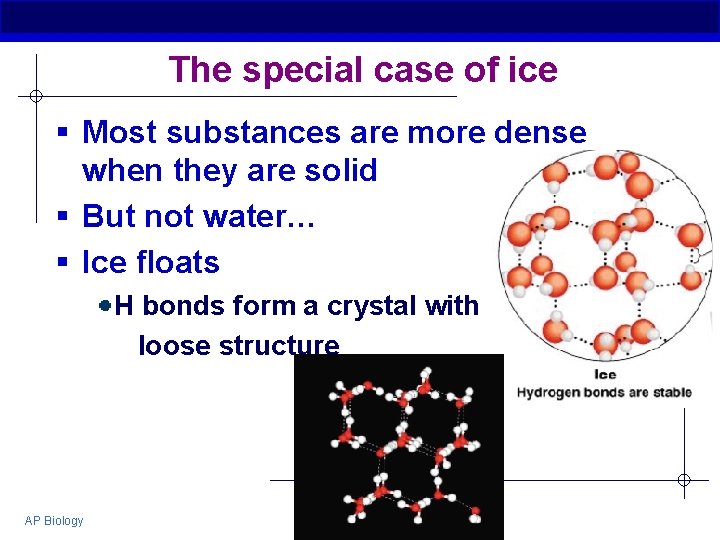
The special case of ice § Most substances are more dense when they are solid § But not water… § Ice floats H bonds form a crystal with loose structure AP Biology
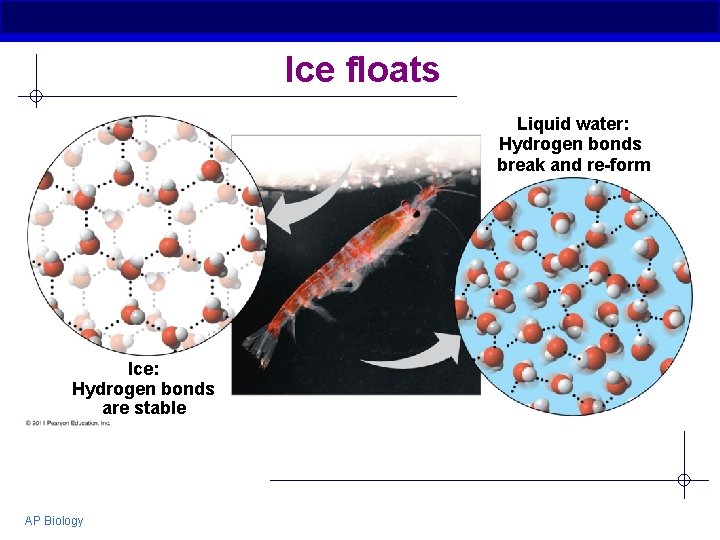
Ice floats Liquid water: Hydrogen bonds break and re-form Ice: Hydrogen bonds are stable AP Biology

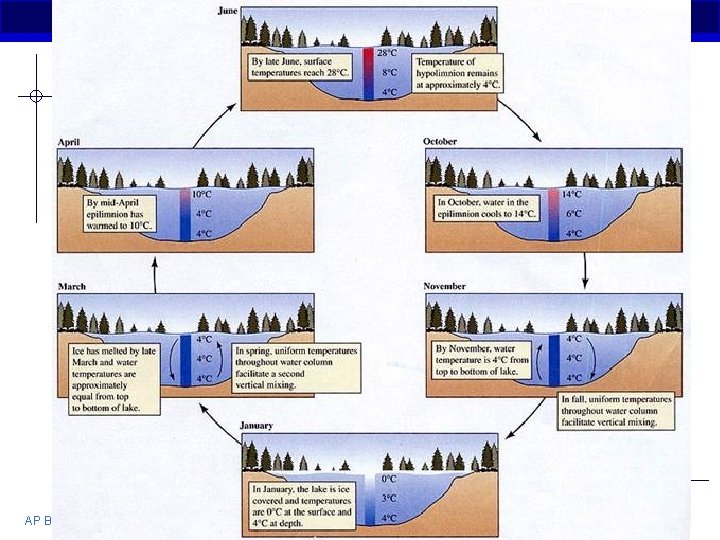
Why is the density of ice important? § Oceans and lakes don’t freeze solid § If ice sank… All ponds, lakes and oceans would eventually freeze solid During summer, only upper few inches would thaw § Surface insulates water below Allows life to survive winter § Seasonal turnover of lakes Cycling nutrients AP Biology
AP Biology

Specific heat § Amount of heat 1 g of a substance must absorb or lose to change its temperature by 1°C § Water has high specific heat Due to H bonding § Water resists changes in temperature Takes a lot to heat it up Takes a lot to cool it down § Water moderates temperatures on earth AP Biology

High Specific Heat § Water will change its temperature less when it absorbs or loses a given amount of heat § In order to change water from solid to a liquid or liquid to a gas: Hydrogen bonds between water molecules must be broken--heat must be absorbed AFTER hydrogen bonds are broken, then water molecules can begin to move faster, thus increasing their kinetic energy The reverse is also true…. AP Biology
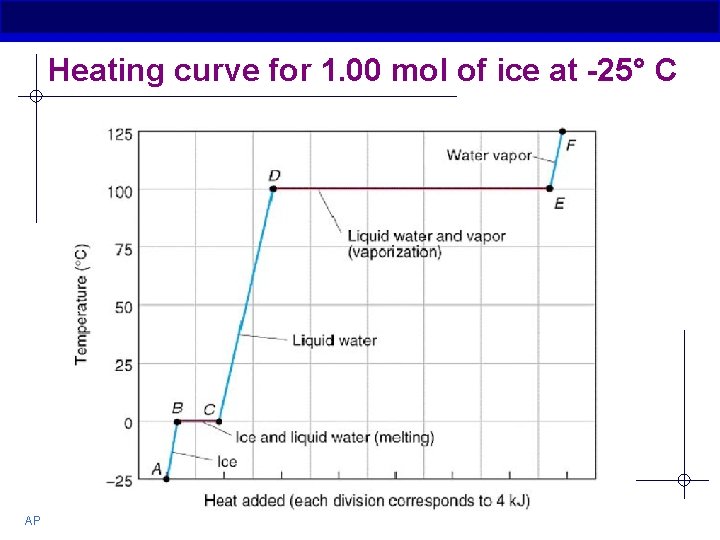
Heating curve for 1. 00 mol of ice at -25° C AP Biology

High Heat of Vaporization § Amount of energy required to change 1 g of substance from liquid to gas § Requires lots of heat energy (586 cal) to accomplish this change in water § Facilitates Cooling § Organisms rely on heat of vaporization to remove heat AP Biology

Why is this important to living things? Large bodies of water absorb and store huge amount of heat in daytime and during warm seasons, without a dramatic increase in temperature At night and during cooler seasons, gradual cooling of water warms the air § Results in coastal areas having milder climates than areas further away from the ocean § Tends to stabilize ocean temperatures § Because organisms are made mostly of water, they are able to resist changes in their own temperatures AP Biology
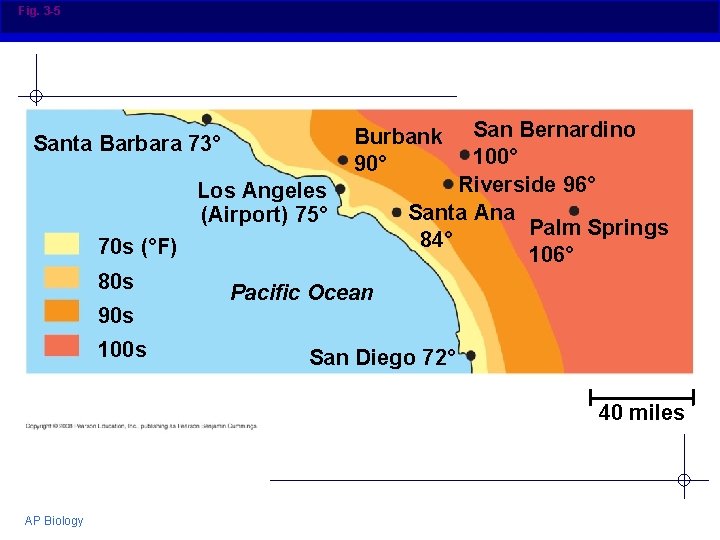
Fig. 3 -5 Los Angeles (Airport) 75° 70 s (°F) 80 s 90 s 100 s San Bernardino 100° Riverside 96° Santa Ana Palm Springs 84° 106° Burbank 90° Santa Barbara 73° Pacific Ocean San Diego 72° 40 miles AP Biology
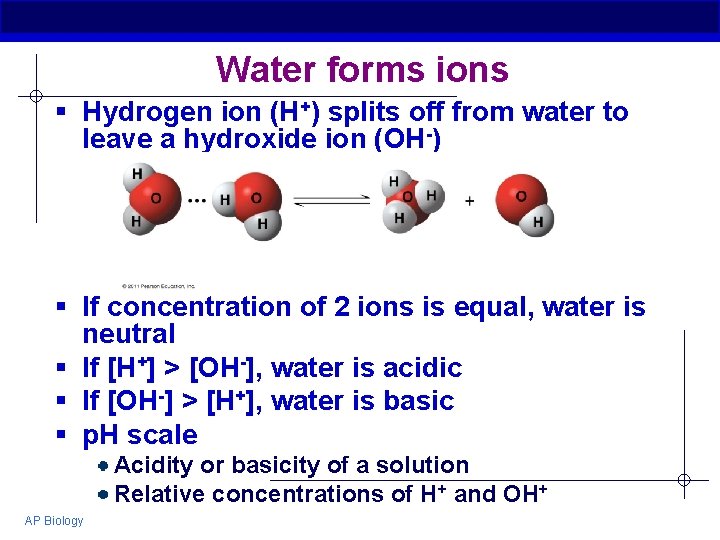
Water forms ions § Hydrogen ion (H+) splits off from water to leave a hydroxide ion (OH-) § If concentration of 2 ions is equal, water is neutral § If [H+] > [OH-], water is acidic § If [OH-] > [H+], water is basic § p. H scale Acidity or basicity of a solution Relative concentrations of H+ and OH+ AP Biology
![Figure 3. UN 05 0 Acidic [H+] > [OH ] Neutral [H+] = [OH Figure 3. UN 05 0 Acidic [H+] > [OH ] Neutral [H+] = [OH](http://slidetodoc.com/presentation_image_h2/83824afa86c3d6967d4d57cd371efa9d/image-24.jpg)
Figure 3. UN 05 0 Acidic [H+] > [OH ] Neutral [H+] = [OH ] Basic [H+] < [OH ] Acids donate H+ in aqueous solutions. 7 Bases donate OH or accept H+ in aqueous solutions 14
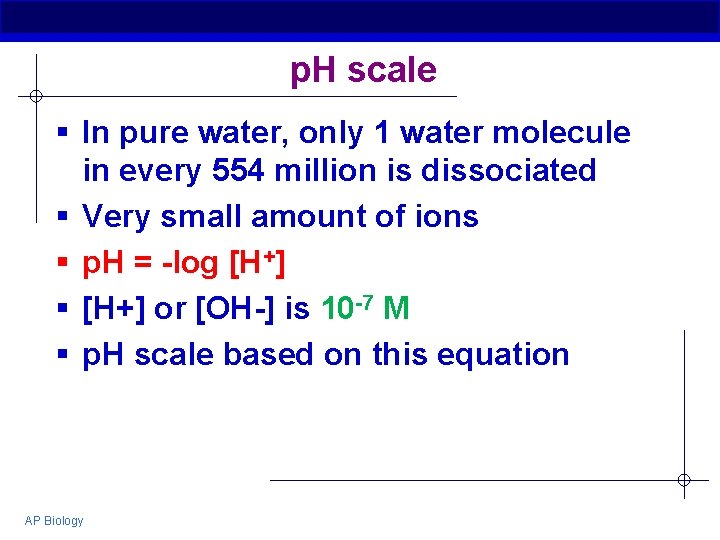
p. H scale § In pure water, only 1 water molecule in every 554 million is dissociated § Very small amount of ions § p. H = -log [H+] § [H+] or [OH-] is 10 -7 M § p. H scale based on this equation AP Biology
p. H and biology § Most biological fluids p. H 6 -8 p. H values in human stomach can reach 2 § Each p. H unit represents a 10 fold difference in H+ & OH- concentrations Small change in p. H actually indicates a substantial change in [H+] and [OH-] AP Biology
Buffers § Living organisms must maintain p. H within a fairly narrow range § Buffer Substance that minimizes changes in [H+] & [OH-] in a solution § Buffers accept H+ when they are in excess & donate H+ when they are depleted AP Biology
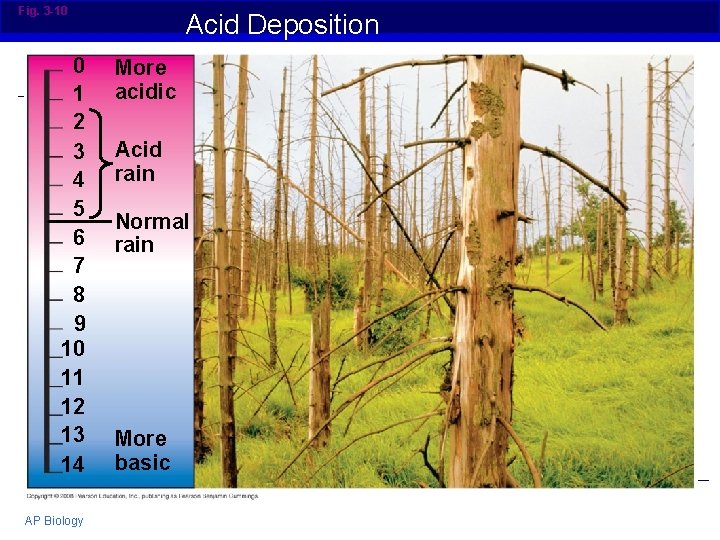
Fig. 3 -10 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 AP Biology Acid Deposition More acidic Acid rain Normal rain More basic
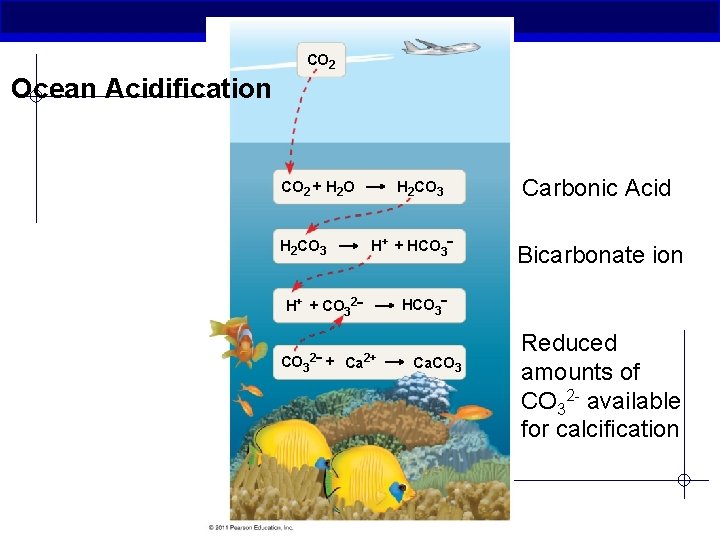
CO 2 Ocean Acidification CO 2 + H 2 O H 2 CO 3 H+ + HCO 3 H 2 CO 3 H+ + CO 32 CO 3 2 + Ca 2+ Carbonic Acid Bicarbonate ion HCO 3 Ca. CO 3 Reduced amounts of CO 32 - available for calcification
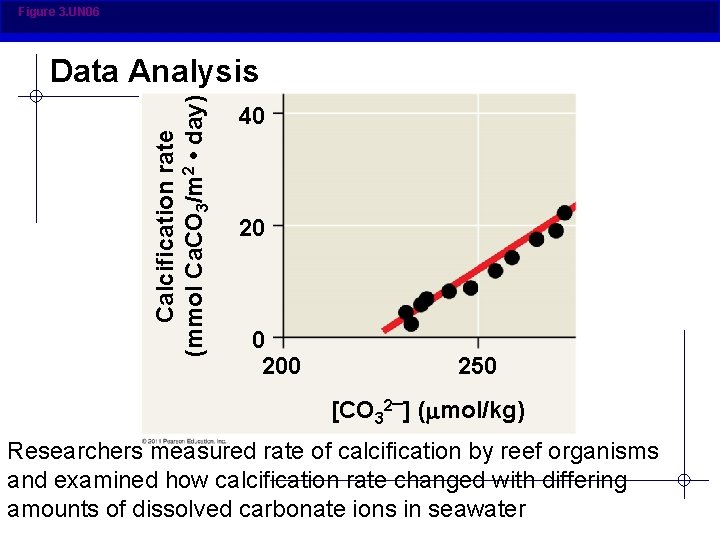
Figure 3. UN 06 Calcification rate (mmol Ca. CO 3/m 2 • day) Data Analysis 40 200 250 [CO 32 ] ( mol/kg) Researchers measured rate of calcification by reef organisms and examined how calcification rate changed with differing amounts of dissolved carbonate ions in seawater
- Slides: 30