Why are electrons important Electrons are responsible for
Why are electrons important? • Electrons are responsible for HOW ELEMENTS REACT • That is why we learned how to: – Write electron configurations – Draw electron orbital diagrams • When we understand electrons – we can predict how elements will behave (what we can do with them) – Understand the structure of the periodic table
Valence Electrons • The specific electrons responsible for the chemical properties of an element • Electrons in an atoms outermost orbitals • They are in the highest energy levels
Valence Electrons • Identify number of valence electrons using… –Electron Shell Diagrams –Electron Configurations –Periodic Table
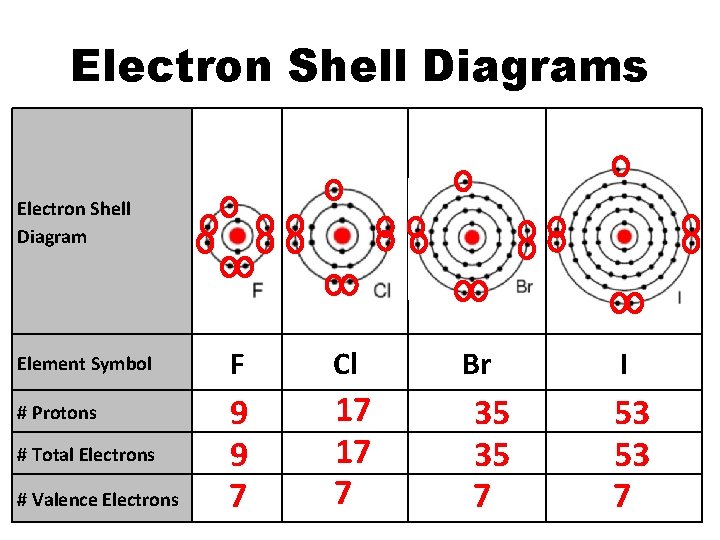
Electron Shell Diagrams Electron Shell Diagram Element Symbol # Protons # Total Electrons # Valence Electrons F Cl 9 9 7 17 17 7 Br 35 35 7 I 53 53 7
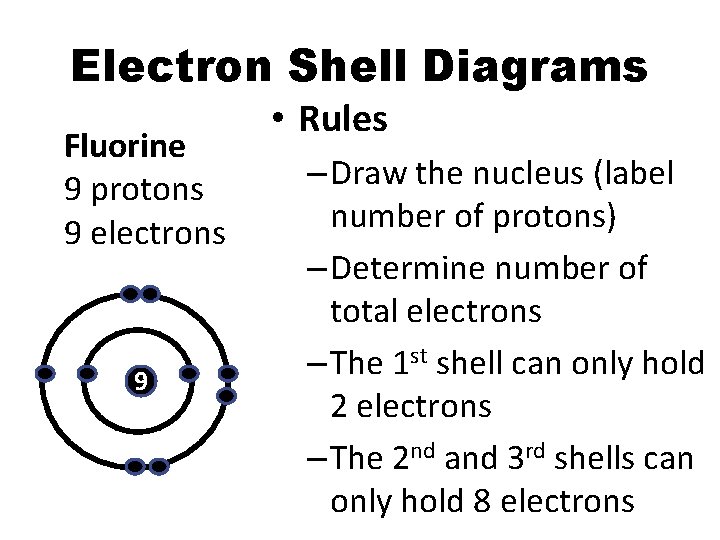
Electron Shell Diagrams Fluorine 9 protons 9 electrons 9 • Rules – Draw the nucleus (label number of protons) – Determine number of total electrons – The 1 st shell can only hold 2 electrons – The 2 nd and 3 rd shells can only hold 8 electrons
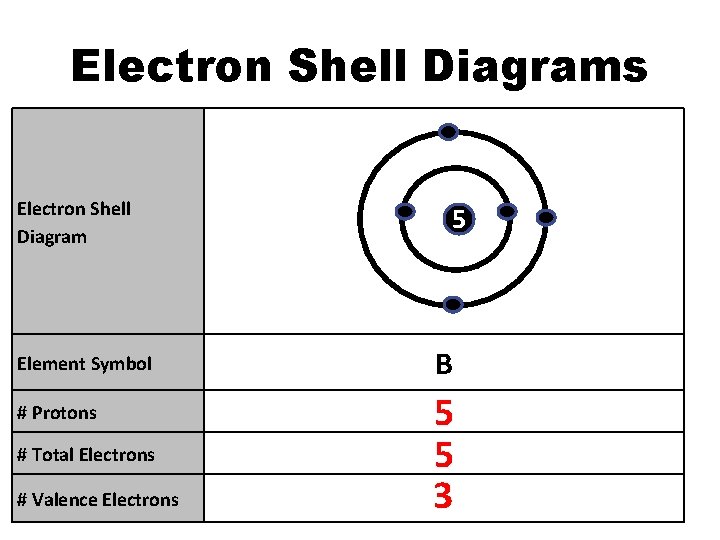
Electron Shell Diagrams Electron Shell Diagram Element Symbol # Protons # Total Electrons # Valence Electrons 5 B 5 5 3
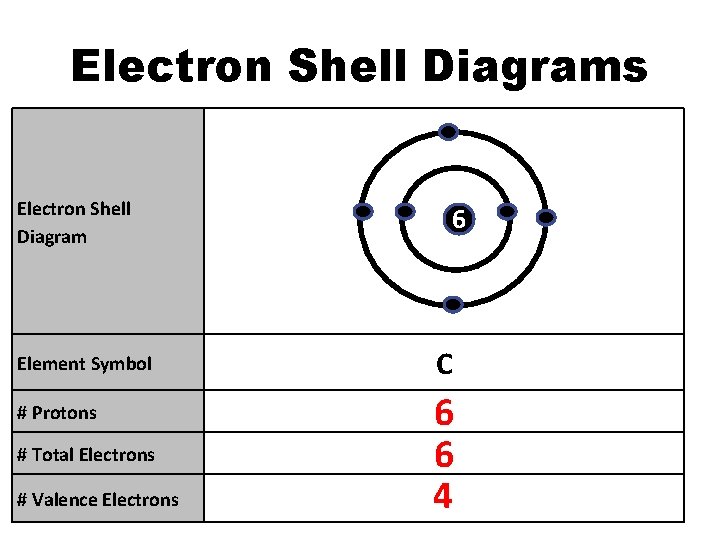
Electron Shell Diagrams Electron Shell Diagram Element Symbol # Protons # Total Electrons # Valence Electrons 6 C 6 6 4
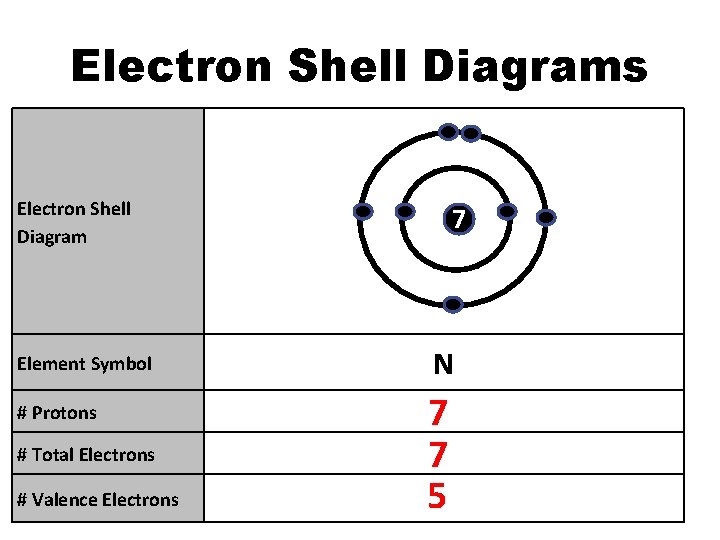
Electron Shell Diagrams Electron Shell Diagram Element Symbol # Protons # Total Electrons # Valence Electrons 7 N 7 7 5
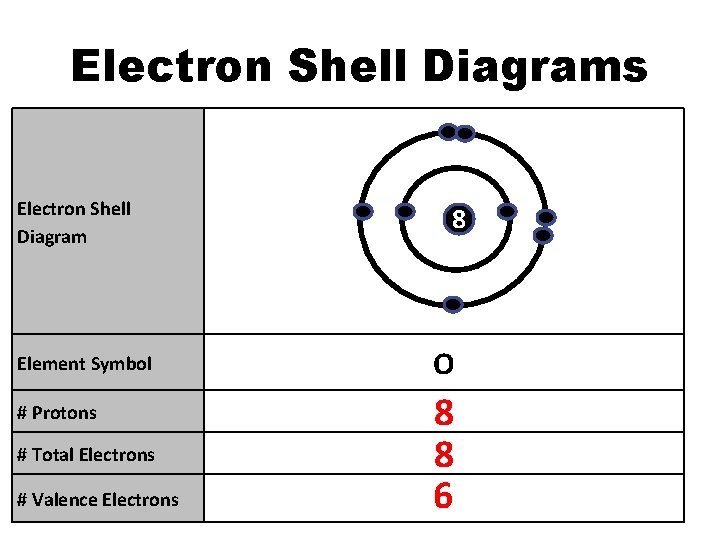
Electron Shell Diagrams Electron Shell Diagram Element Symbol # Protons # Total Electrons # Valence Electrons 8 O 8 8 6
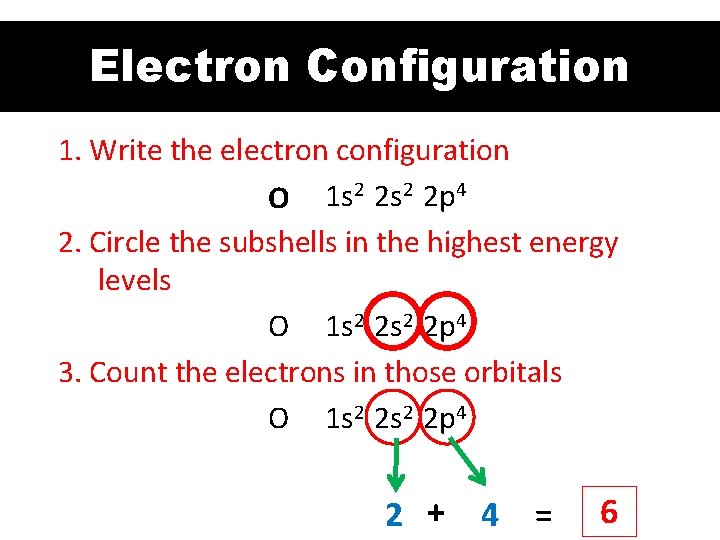
Electron Configuration 1. Write the electron configuration O 1 s 2 2 p 4 2. Circle the subshells in the highest energy levels O 1 s 2 2 p 4 3. Count the electrons in those orbitals O 1 s 2 2 p 4 2 + 4 = 6
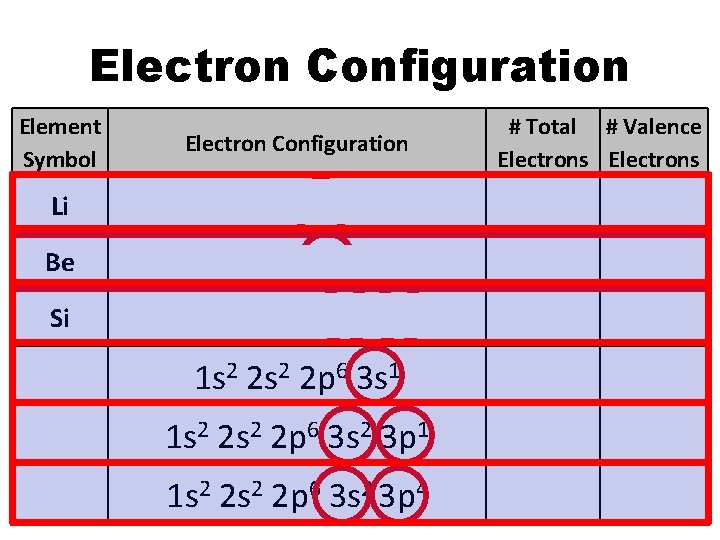
Electron Configuration Element Symbol # Total # Valence Electrons Electron Configuration Li 1 s 2 2 s 1 3 1 Be 1 s 2 2 s 2 4 2 Si 1 s 2 2 p 6 3 s 2 3 p 2 14 4 Na 1 s 2 2 p 6 3 s 1 11 1 Al 1 s 2 2 p 6 3 s 2 3 p 1 13 3 S 1 s 2 2 p 6 3 s 2 3 p 4 16 6
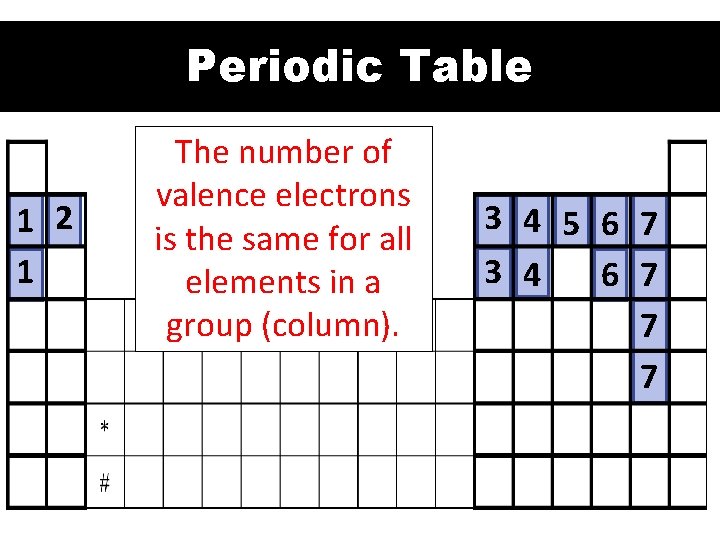
Periodic Table 1 2 1 The number of valence electrons is the same for all elements in a group (column). 3 4 5 6 7 3 4 6 7 7 7
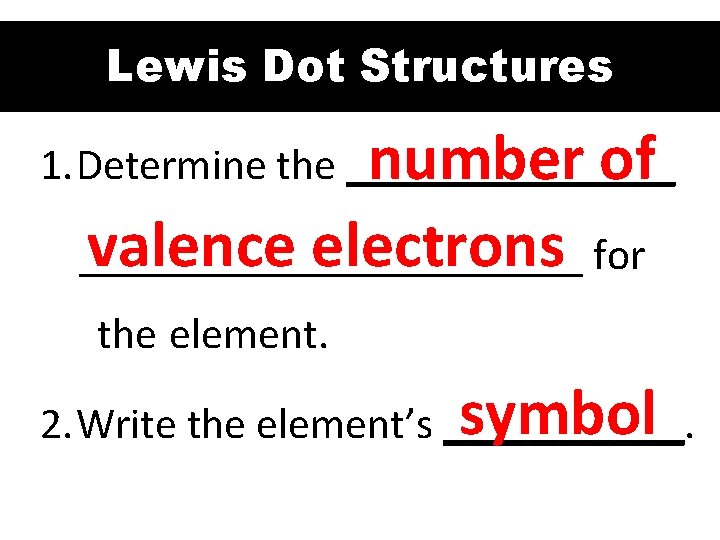
Lewis Dot Structures number of 1. Determine the ________ valence electrons for ____________ the element. symbol 2. Write the element’s ______.
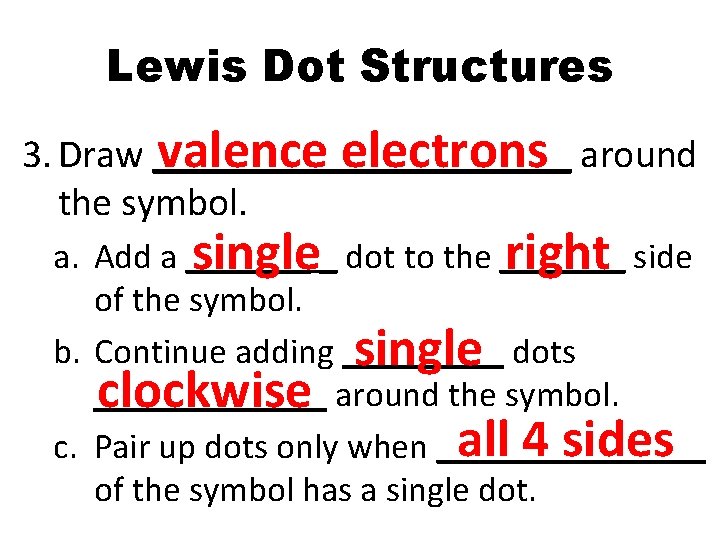
Lewis Dot Structures 3. Draw ___________ valence electrons around the symbol. a. Add a _______ single_ dot to the _______ right side of the symbol. b. Continue adding _____ single dots _______ clockwise around the symbol. all 4 sides c. Pair up dots only when ________ of the symbol has a single dot.
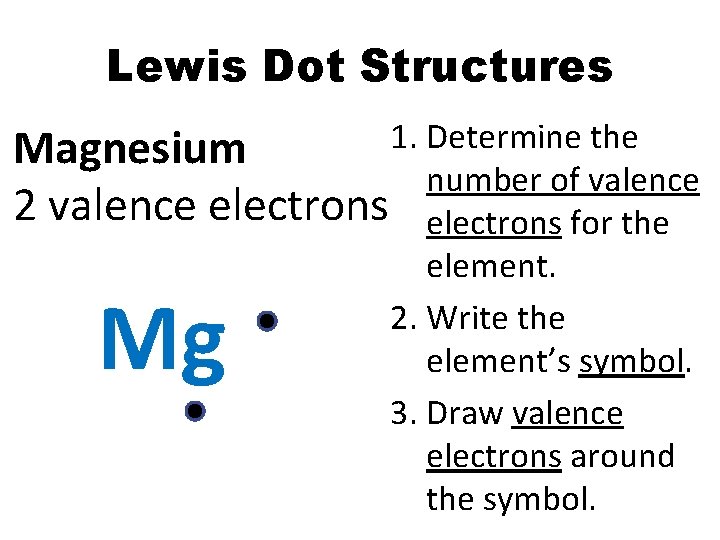
Lewis Dot Structures 1. Determine the Magnesium number of valence 2 valence electrons for the element. 2. Write the element’s symbol. 3. Draw valence electrons around the symbol. Mg
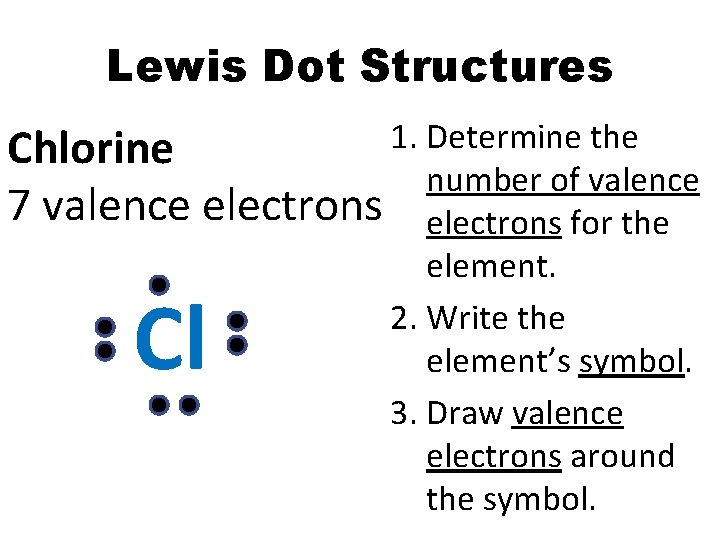
Lewis Dot Structures 1. Determine the Chlorine number of valence 7 valence electrons for the element. 2. Write the element’s symbol. 3. Draw valence electrons around the symbol. Cl
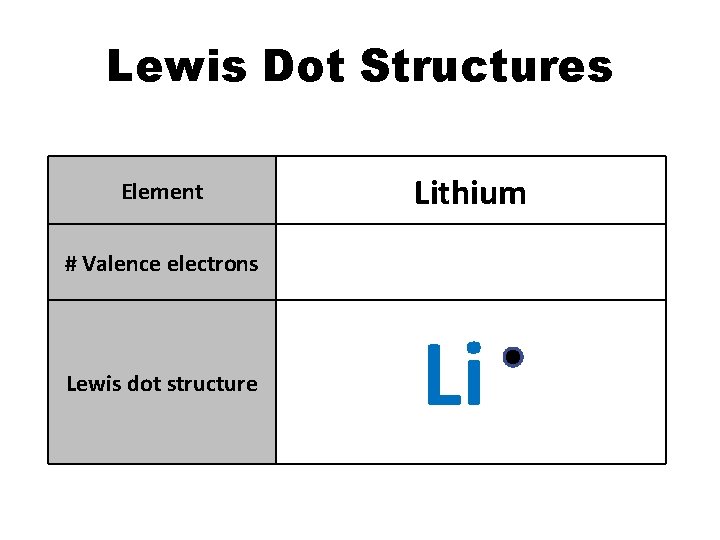
Lewis Dot Structures Element Lithium # Valence electrons 1 Lewis dot structure Li
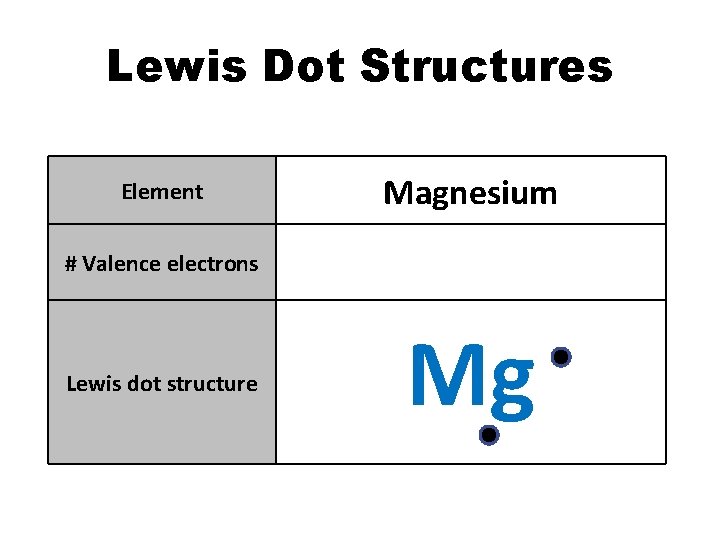
Lewis Dot Structures Element Magnesium # Valence electrons 2 Lewis dot structure Mg
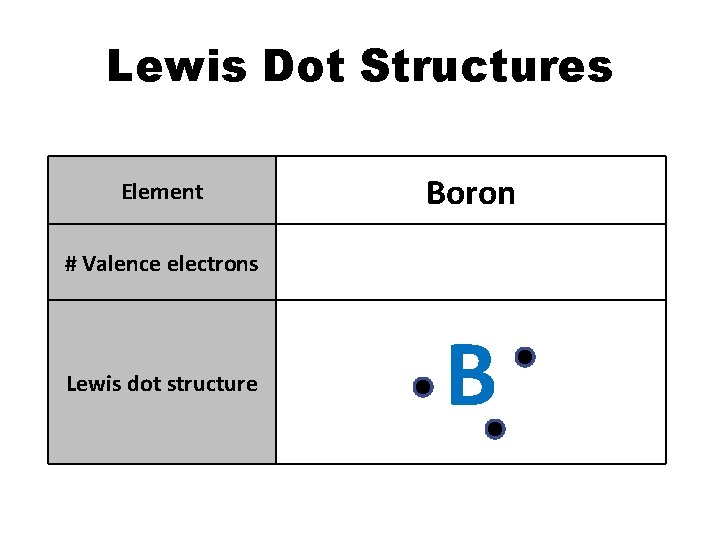
Lewis Dot Structures Element Boron # Valence electrons 3 Lewis dot structure B
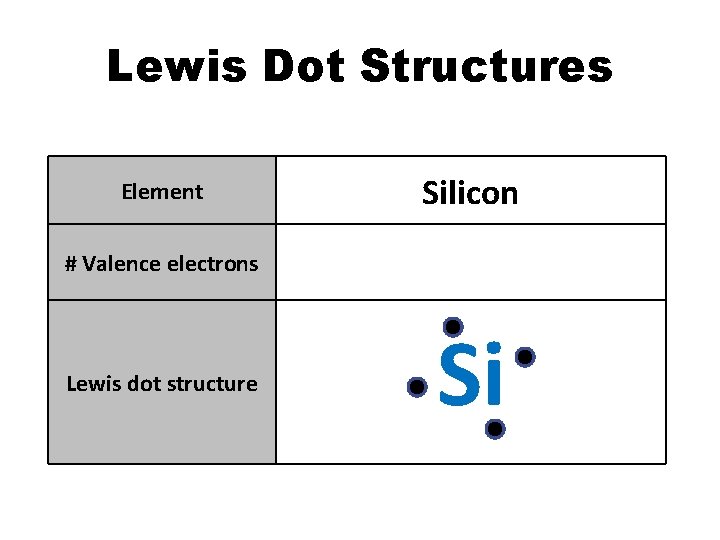
Lewis Dot Structures Element Silicon # Valence electrons 4 Lewis dot structure Si
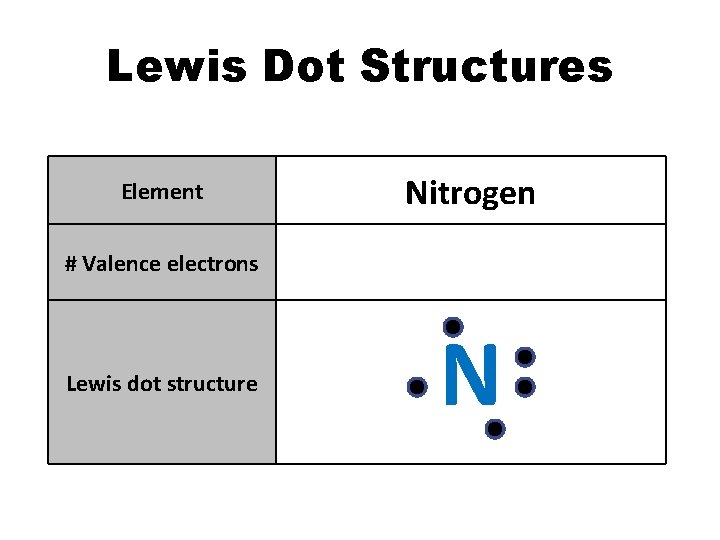
Lewis Dot Structures Element Nitrogen # Valence electrons 5 Lewis dot structure N
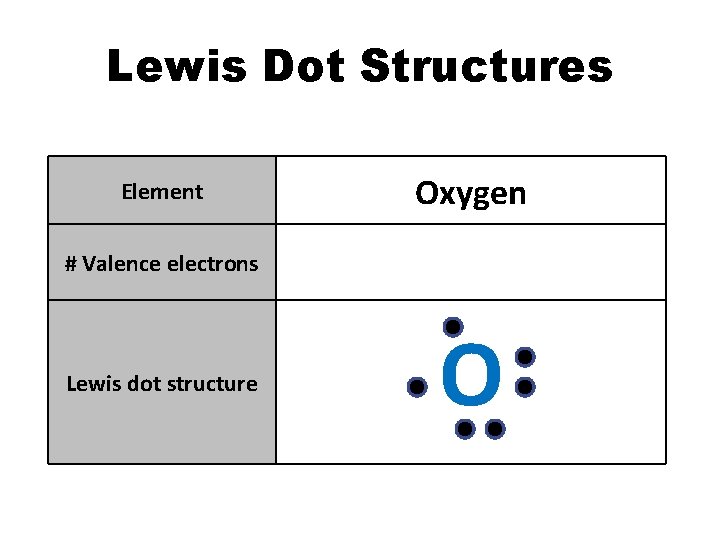
Lewis Dot Structures Element Oxygen # Valence electrons 6 Lewis dot structure O
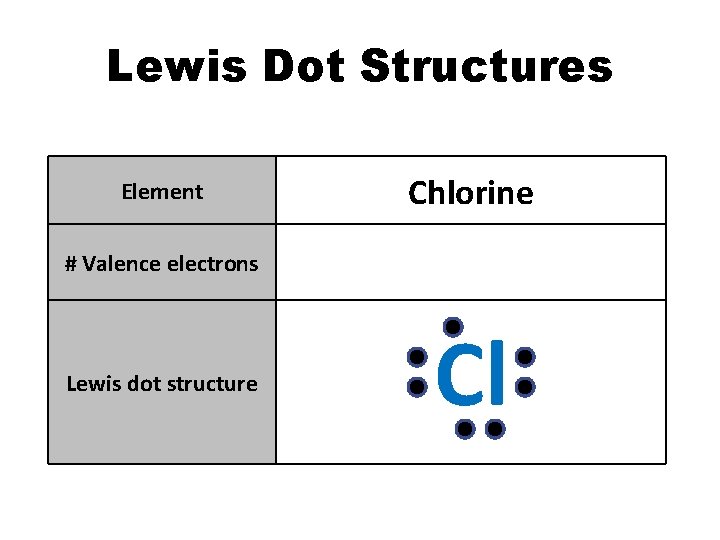
Lewis Dot Structures Element Chlorine # Valence electrons 7 Lewis dot structure Cl
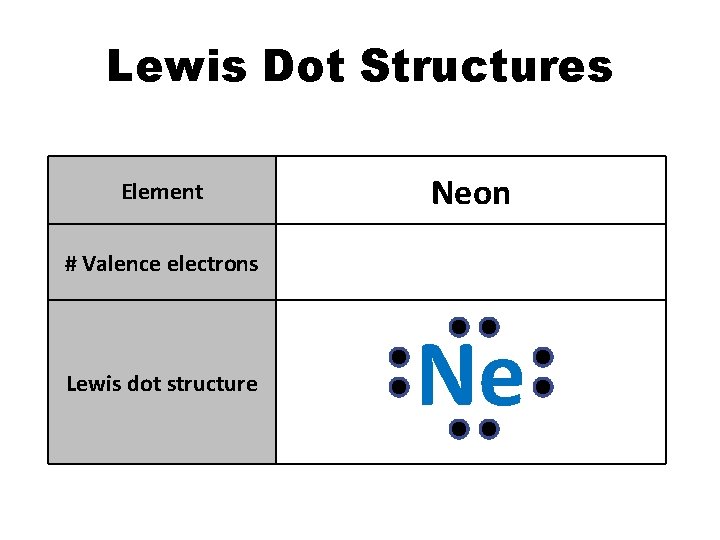
Lewis Dot Structures Element Neon # Valence electrons 8 Lewis dot structure Ne
- Slides: 24