Whom to treat and how long to treat















- Slides: 36

Whom to treat and how long to treat after resection of GIST Professor John R Zalcberg Chief Medical Officer & Director, Division of Cancer Medicine Peter Mac. Callum Cancer Centre Chair, Australasian Gastro-Intestinal Trials Group

Disclosures Research / Travel Support / Advisory Board Ø Novartis Ø Pfizer Ø Bayer Ø Amgen Ø BMS

Risk of Recurrence After Resection of Primary GIST Approximately 40% of patients who undergo complete resection of primary GIST have a recurrence within 5 years De. Matteo RP et al. Cancer. 2008; 112: 608 -615.

Risk Assessment Ø Accurate assessment of risk of aggressive malignant behaviour in GIST poses a challenge 1 Ø Morphologic features most predictive of outcome 1, 2 - Mitotic index - Tumour size Ø Tumour site and rupture also affect risk of recurrence and progression 2, 3 Ø Mutational status is useful in predicting treatment response in the metastatic setting 4, 5 Ø ? applicable in the adjuvant setting 1. Fletcher CD et al. Hum Pathol. 2002; 33: 459 -465. 2. Demetri GD et al. J Natl Compr Cancer Netw. 2007; 5(suppl 2): S 1 -S 29. 3. Miettinen M, Lasota J. Arch Pathol Lab Med. 2006; 130: 1466 -1478. 4. Debiec-Rychter M et al. Eur J Cancer. 2006; 42: 1093 -1103. 5. Heinrich MC et al. J Clin Oncol. 2003; 21: 4342 -4349.

Primary GIST: Risk Factors for Recurrence After Surgery Rates of RFS were independently predicted by mitotic index, tumour size, and tumour location Adapted with permission from De. Matteo RP et al. Cancer. 2008; 112: 608 -615.

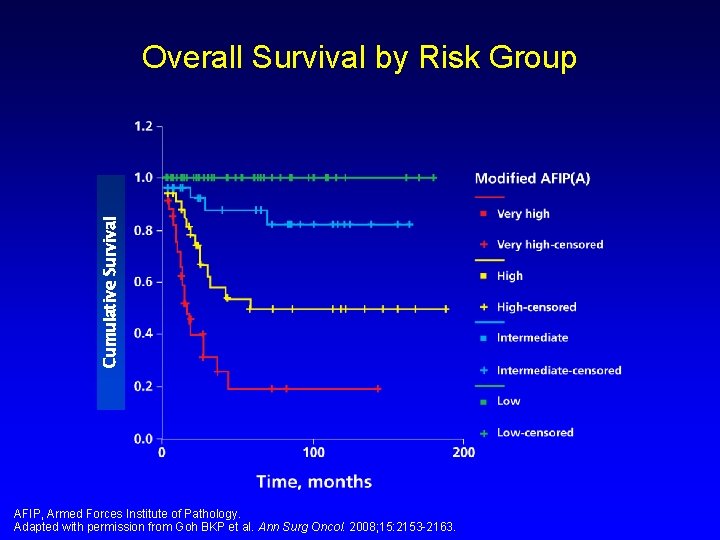
Cumulative Survival Overall Survival by Risk Group AFIP, Armed Forces Institute of Pathology. Adapted with permission from Goh BKP et al. Ann Surg Oncol. 2008; 15: 2153 -2163.

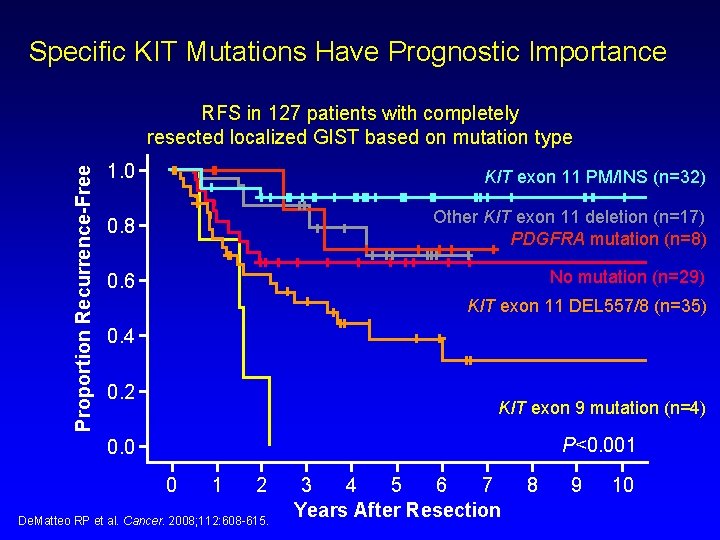
Specific KIT Mutations Have Prognostic Importance Proportion Recurrence-Free RFS in 127 patients with completely resected localized GIST based on mutation type 1. 0 KIT exon 11 PM/INS (n=32) 0. 8 Other KIT exon 11 deletion (n=17) PDGFRA mutation (n=8) 0. 6 No mutation (n=29) KIT exon 11 DEL 557/8 (n=35) 0. 4 0. 2 KIT exon 9 mutation (n=4) P<0. 001 0. 0 0 1 2 De. Matteo RP et al. Cancer. 2008; 112: 608 -615. 3 4 5 6 7 Years After Resection 8 9 10

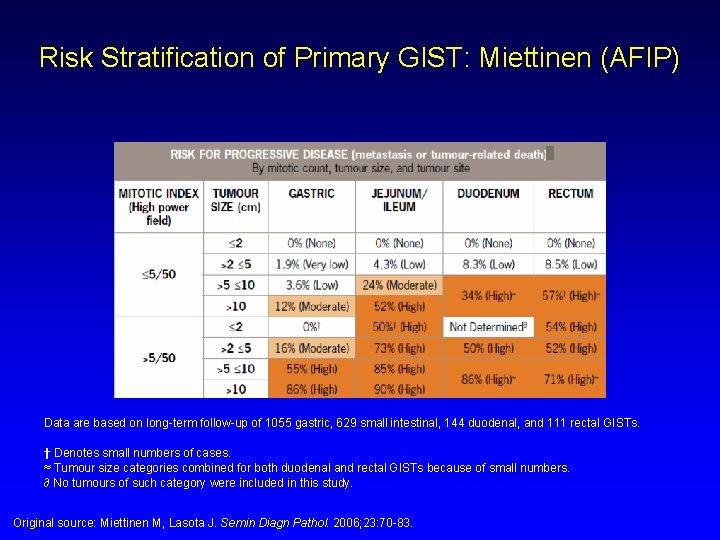
Risk Stratification of Primary GIST: Miettinen (AFIP) Data are based on long-term follow-up of 1055 gastric, 629 small intestinal, 144 duodenal, and 111 rectal GISTs. † Denotes small numbers of cases. ≈ Tumour size categories combined for both duodenal and rectal GISTs because of small numbers. ∂ No tumours of such category were included in this study. Original source: Miettinen M, Lasota J. Semin Diagn Pathol. 2006; 23: 70 -83.

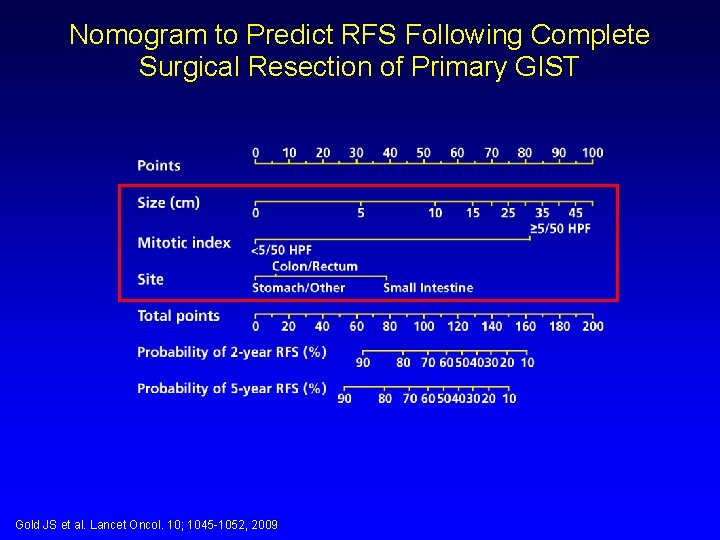
Nomogram to Predict RFS Following Complete Surgical Resection of Primary GIST Gold JS et al. Lancet Oncol. 10; 1045 -1052, 2009

Risk Classification – Room for Refinement Ø Additional factors: - Mutational status 1 - Rupture 2 - Necrosis 3 1. Wardelmann E et al. Virchoves Arch. 2007; 451: 743 -749. 2. Joensuu H. Hum Path. 2008; 39: 1411 -1419. 3. Dei Tos AP et al. J Clin Oncol. 2009; 27(suppl). Abstract 10555.

Joensuu H et al. Lancet Oncol, 13; 265 -274, 2012

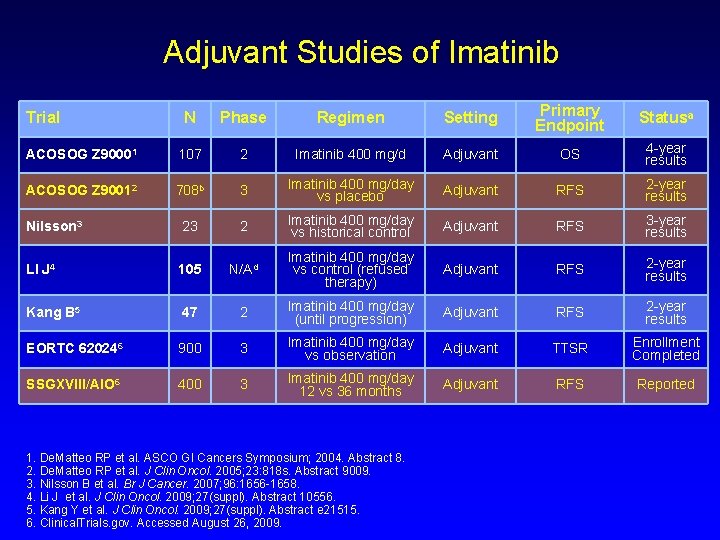
Adjuvant Studies of Imatinib N Phase Regimen Setting Primary Endpoint Statusa ACOSOG Z 90001 107 2 Imatinib 400 mg/d Adjuvant OS 4 -year results ACOSOG Z 90012 708 b 3 Imatinib 400 mg/day vs placebo Adjuvant RFS 2 -year results Nilsson 3 23 2 Imatinib 400 mg/day vs historical control Adjuvant RFS 3 -year results LI J 4 105 N/Ad Imatinib 400 mg/day vs control (refused therapy) Adjuvant RFS 2 -year results Kang B 5 47 2 Imatinib 400 mg/day (until progression) Adjuvant RFS 2 -year results EORTC 620246 900 3 Imatinib 400 mg/day vs observation Adjuvant TTSR Enrollment Completed SSGXVIII/AIO 6 400 3 Imatinib 400 mg/day 12 vs 36 months Adjuvant RFS Reported Trial 1. De. Matteo RP et al. ASCO GI Cancers Symposium; 2004. Abstract 8. 2. De. Matteo RP et al. J Clin Oncol. 2005; 23: 818 s. Abstract 9009. 3. Nilsson B et al. Br J Cancer. 2007; 96: 1656 -1658. 4. Li J et al. J Clin Oncol. 2009; 27(suppl). Abstract 10556. 5. Kang Y et al. J Clin Oncol. 2009; 27(suppl). Abstract e 21515. 6. Clinical. Trials. gov. Accessed August 26, 2009.

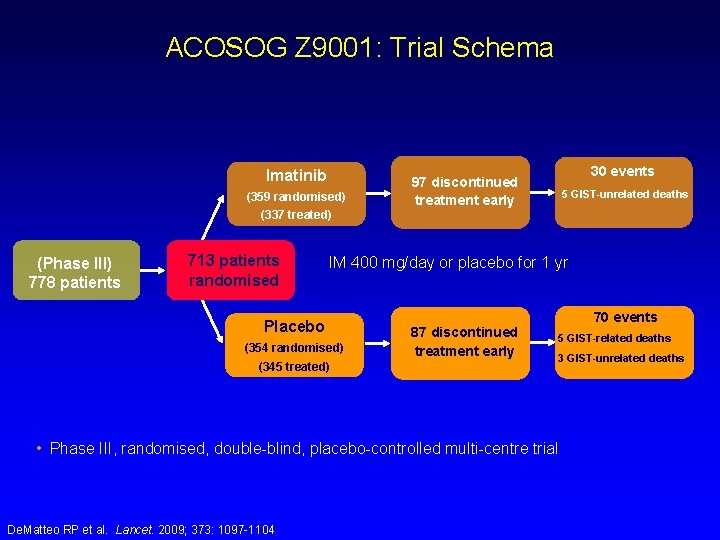
ACOSOG Z 9001: Trial Schema Imatinib (359 randomised) 30 events 97 discontinued treatment early 5 GIST-unrelated deaths (337 treated) (Phase III) 778 patients 713 patients randomised IM 400 mg/day or placebo for 1 yr Placebo (354 randomised) (345 treated) 70 events 87 discontinued treatment early 5 GIST-related deaths 3 GIST-unrelated deaths • Phase III, randomised, double-blind, placebo-controlled multi-centre trial De. Matteo RP et al. Lancet. 2009; 373: 1097 -1104

ACOSOG Z 9001: Study Design/Methods Key Eligibility Criteria: • Patients ≥ 18 years with localised and primary GIST • KIT-positive tumours ≥ 3 cm • Complete surgical resection Endpoints: • Primary: Recurrence-free Survival (RFS) • Secondary: Overall Survival (OS) and safety Other Key Elements: • Dose modifications upon grade 3 or 4 events • PD patients unblinded: - If placebo IM 400 mg/day or - If IM 400 mg/day IM 800 mg/day

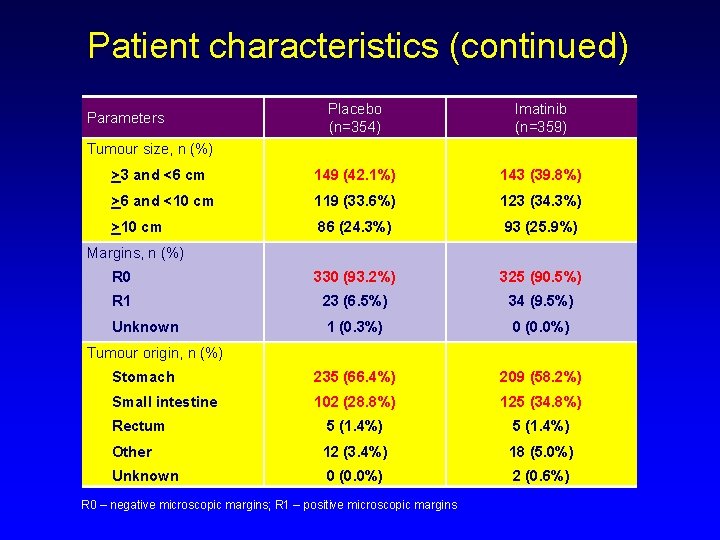
Patient characteristics (continued) Placebo (n=354) Imatinib (n=359) >3 and <6 cm 149 (42. 1%) 143 (39. 8%) >6 and <10 cm 119 (33. 6%) 123 (34. 3%) >10 cm 86 (24. 3%) 93 (25. 9%) R 0 330 (93. 2%) 325 (90. 5%) R 1 23 (6. 5%) 34 (9. 5%) Unknown 1 (0. 3%) 0 (0. 0%) Stomach 235 (66. 4%) 209 (58. 2%) Small intestine 102 (28. 8%) 125 (34. 8%) Rectum 5 (1. 4%) Other 12 (3. 4%) 18 (5. 0%) Unknown 0 (0. 0%) 2 (0. 6%) Parameters Tumour size, n (%) Margins, n (%) Tumour origin, n (%) R 0 – negative microscopic margins; R 1 – positive microscopic margins

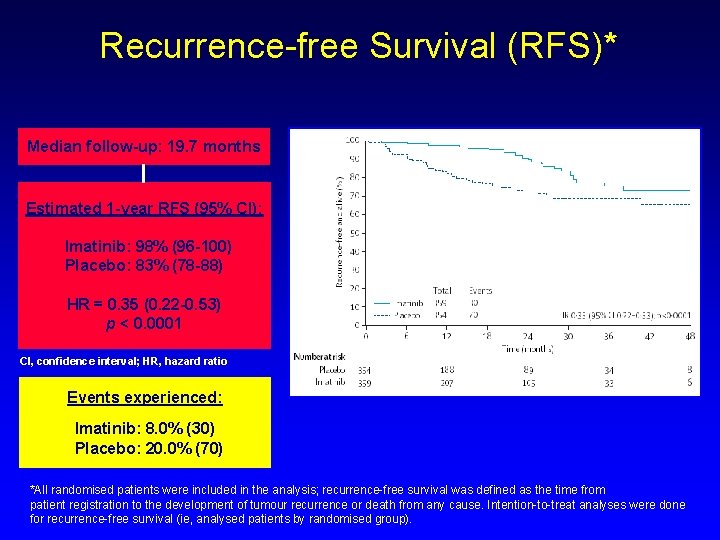
Recurrence-free Survival (RFS)* Median follow-up: 19. 7 months Estimated 1 -year RFS (95% CI): Imatinib: 98% (96 -100) Placebo: 83% (78 -88) HR = 0. 35 (0. 22 -0. 53) p < 0. 0001 CI, confidence interval; HR, hazard ratio Events experienced: Imatinib: 8. 0% (30) Placebo: 20. 0% (70) *All randomised patients were included in the analysis; recurrence-free survival was defined as the time from patient registration to the development of tumour recurrence or death from any cause. Intention-to-treat analyses were done for recurrence-free survival (ie, analysed patients by randomised group).

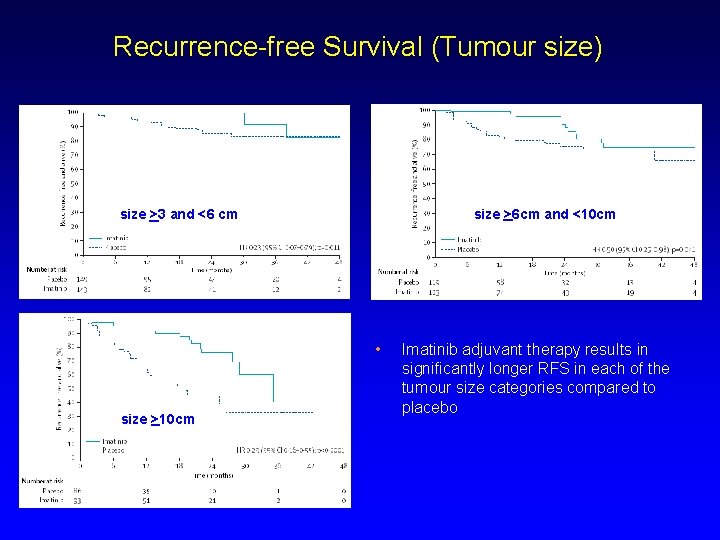
Recurrence-free Survival (Tumour size) size >6 cm and <10 cm size >3 and <6 cm • size >10 cm Imatinib adjuvant therapy results in significantly longer RFS in each of the tumour size categories compared to placebo

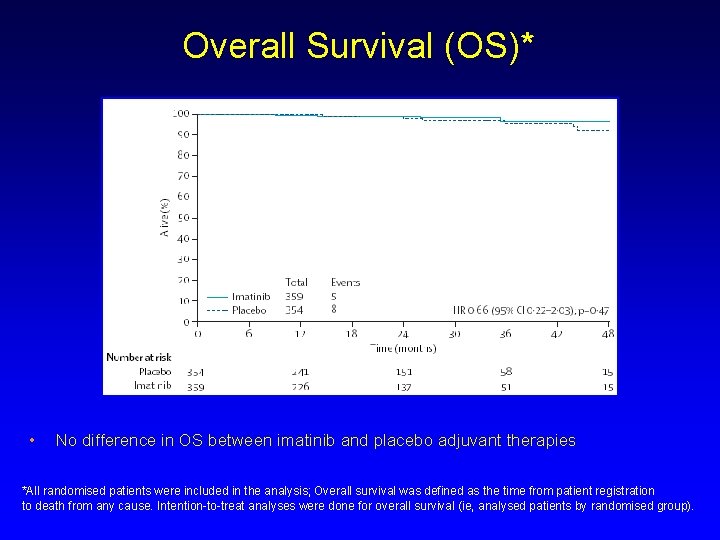
Overall Survival (OS)* • No difference in OS between imatinib and placebo adjuvant therapies *All randomised patients were included in the analysis; Overall survival was defined as the time from patient registration to death from any cause. Intention-to-treat analyses were done for overall survival (ie, analysed patients by randomised group).

Summary Ø Imatinib at 400 mg/day is safe and well tolerated when administered as adjuvant therapy after complete resection of primary GIST Ø Adjuvant imatinib resulted in an improvement in RFS in patients with all tumour sizes - Especially relevant for high-risk patients (e. g. tumour size ≥ 10 cm or high mitotic rate) since this patient population has a 50% higher chance of recurrence at 2 years without adjuvant therapy Ø OS between imatinib and placebo groups comparable at this time Ø A longer follow-up period is likely required to observe differences Ø Ongoing trials in the adjuvant setting are under way to determine appropriate treatment duration of imatinib and impact on OS – SSGXVIII/AIO – EORTC 62024

Adjuvant Imatinib: Beyond 1 Year of Treatment

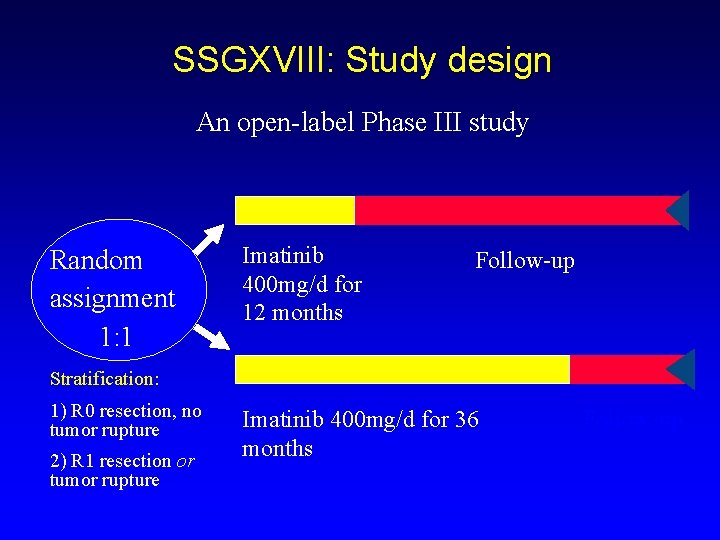
SSGXVIII: Study design An open-label Phase III study Random assignment 1: 1 Imatinib 400 mg/d for 12 months Follow-up Stratification: 1) R 0 resection, no tumor rupture 2) R 1 resection or tumor rupture Imatinib 400 mg/d for 36 months Follow-up

SSGXVIII: Objectives Ø Primary: RFS Time from randomization to GIST recurrence or death Ø Secondary objectives included: Safety Overall survival

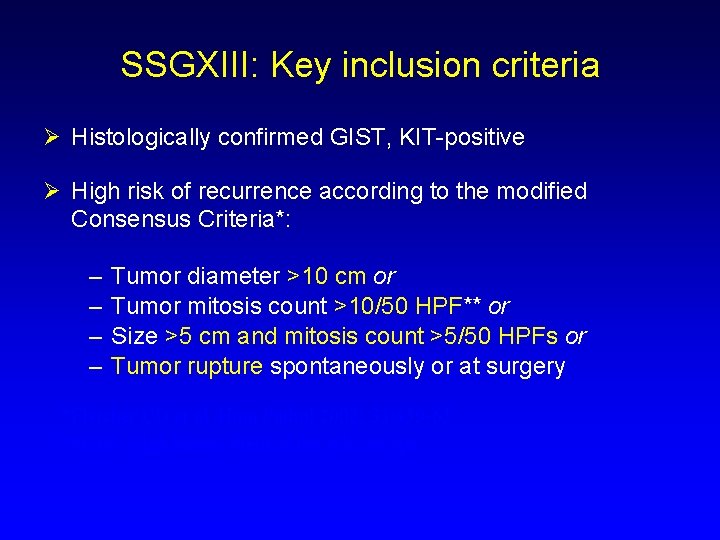
SSGXIII: Key inclusion criteria Ø Histologically confirmed GIST, KIT-positive Ø High risk of recurrence according to the modified Consensus Criteria*: – – Tumor diameter >10 cm or Tumor mitosis count >10/50 HPF** or Size >5 cm and mitosis count >5/50 HPFs or Tumor rupture spontaneously or at surgery *Fletcher CD et al. Hum Pathol 2002; 33: 459 -65 **HPF, High Power Field of the microscope

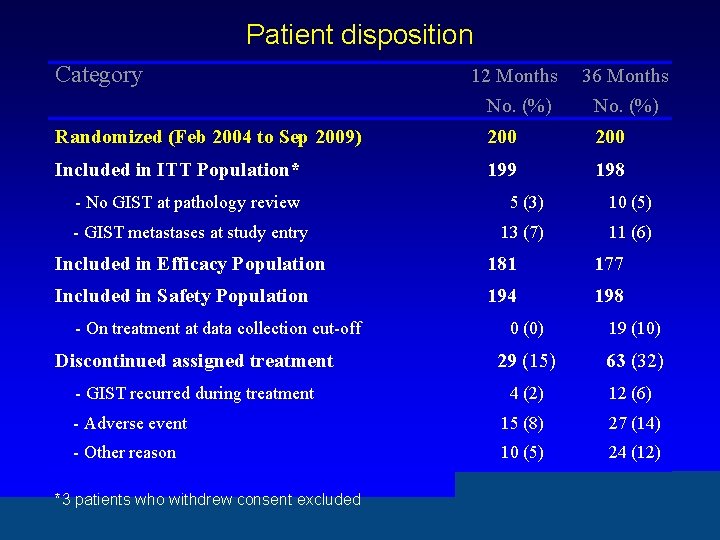
Patient disposition Category 12 Months No. (%) 36 Months No. (%) Randomized (Feb 2004 to Sep 2009) 200 Included in ITT Population* 199 198 - No GIST at pathology review 5 (3) 10 (5) - GIST metastases at study entry 13 (7) 11 (6) Included in Efficacy Population 181 177 Included in Safety Population 194 198 - On treatment at data collection cut-off 0 (0) 19 (10) Discontinued assigned treatment 29 (15) 63 (32) - GIST recurred during treatment 4 (2) 12 (6) - Adverse event 15 (8) 27 (14) - Other reason 10 (5) 24 (12) *3 patients who withdrew consent excluded

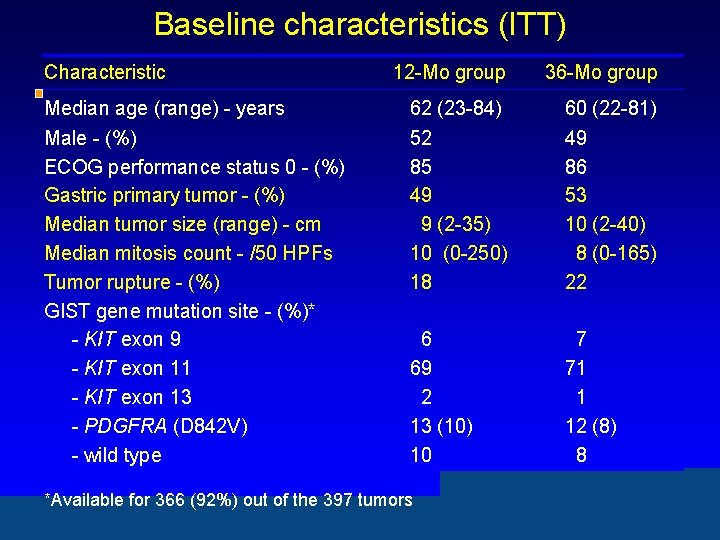
Baseline characteristics (ITT) Characteristic 12 -Mo group 36 -Mo group Median age (range) - years 62 (23 -84) 60 (22 -81) Male - (%) ECOG performance status 0 - (%) Gastric primary tumor - (%) Median tumor size (range) - cm Median mitosis count - /50 HPFs Tumor rupture - (%) GIST gene mutation site - (%)* - KIT exon 9 - KIT exon 11 - KIT exon 13 - PDGFRA (D 842 V) - wild type 52 85 49 9 (2 -35) 10 (0 -250) 18 49 86 53 10 (2 -40) 8 (0 -165) 22 6 69 2 13 (10) 10 7 71 1 12 (8) 8 *Available for 366 (92%) out of the 397 tumors

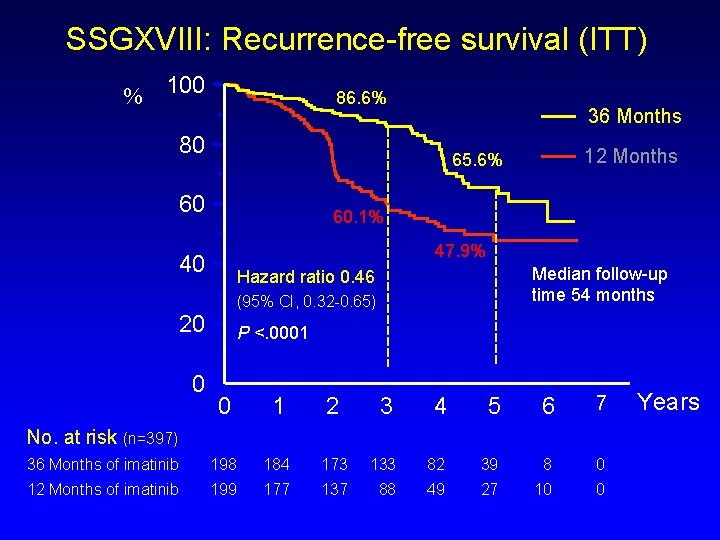
SSGXVIII: Recurrence-free survival (ITT) % 100 86. 6% 36 Months 80 60 60. 1% 47. 9% 40 Median follow-up time 54 months Hazard ratio 0. 46 (95% CI, 0. 32 -0. 65) 20 0 12 Months 65. 6% P <. 0001 0 1 2 3 4 5 6 7 36 Months of imatinib 198 184 173 133 82 39 8 0 12 Months of imatinib 199 177 137 88 49 27 10 0 No. at risk (n=397) Years

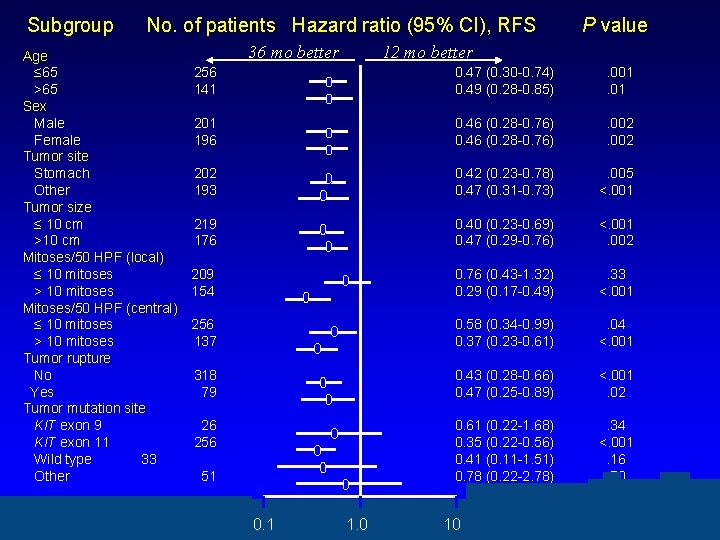
Subgroup No. of patients Hazard ratio (95% CI), RFS Age ≤ 65 256 >65 141 Sex Male 201 Female 196 Tumor site Stomach 202 Other 193 Tumor size ≤ 10 cm 219 >10 cm 176 Mitoses/50 HPF (local) ≤ 10 mitoses 209 > 10 mitoses 154 Mitoses/50 HPF (central) ≤ 10 mitoses 256 > 10 mitoses 137 Tumor rupture No 318 Yes 79 Tumor mutation site KIT exon 9 26 KIT exon 11 256 Wild type 33 Other 51 36 mo better 0. 1 P value 12 mo better 1. 0 0. 47 (0. 30 -0. 74) 0. 49 (0. 28 -0. 85) . 001. 01 0. 46 (0. 28 -0. 76) . 002 0. 42 (0. 23 -0. 78) 0. 47 (0. 31 -0. 73) . 005 <. 001 0. 40 (0. 23 -0. 69) 0. 47 (0. 29 -0. 76) <. 001. 002 0. 76 (0. 43 -1. 32) 0. 29 (0. 17 -0. 49) . 33 <. 001 0. 58 (0. 34 -0. 99) 0. 37 (0. 23 -0. 61) . 04 <. 001 0. 43 (0. 28 -0. 66) 0. 47 (0. 25 -0. 89) <. 001. 02 0. 61 (0. 22 -1. 68) 0. 35 (0. 22 -0. 56) 0. 41 (0. 11 -1. 51) 0. 78 (0. 22 -2. 78) . 34 <. 001. 16. 70 10 10

Clinical Risk Factors and Risk-Reduction with 3 Years of Adjuvant Imatinib Risk Factor No. Patients Hazard Ratio (95% CI, RFS) P-Value TUMOUR SITE Gastric 202 0. 42 (0. 23 -0. 78) 0. 006 Non-Gastric 195 0. 47(0. 31 -0. 73) <0. 001 <10 cm. 219 0. 40 (0. 24 -0. 69) <0. 001 >10 cm. 176 0. 47 (0. 29 -0. 76) 0. 002 <10 238 0. 53 (0. 30 -0. 94) 0. 03 >10 133 0. 36 (0. 22 -0. 59) <0. 001 SIZE Mitoses/50 HPF

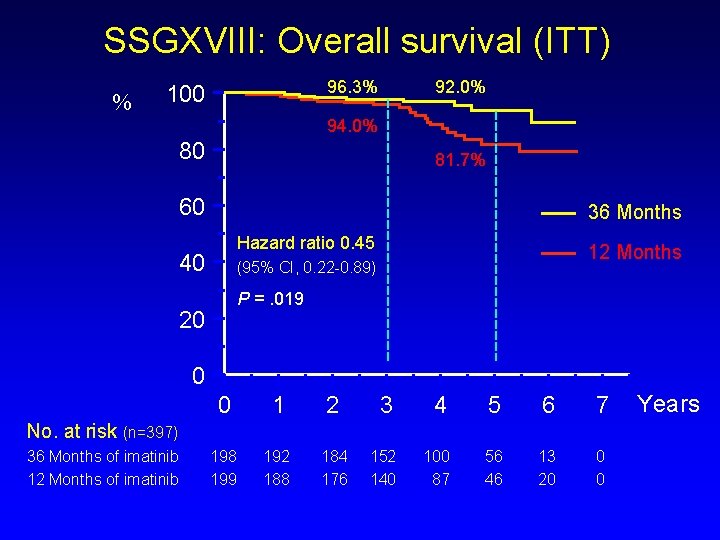
SSGXVIII: Overall survival (ITT) % 96. 3% 100 92. 0% 94. 0% 80 81. 7% 60 36 Months Hazard ratio 0. 45 40 12 Months (95% CI, 0. 22 -0. 89) P =. 019 20 0 0 1 2 3 4 5 6 7 198 199 192 188 184 176 152 140 100 87 56 46 13 20 0 0 No. at risk (n=397) 36 Months of imatinib 12 Months of imatinib Years

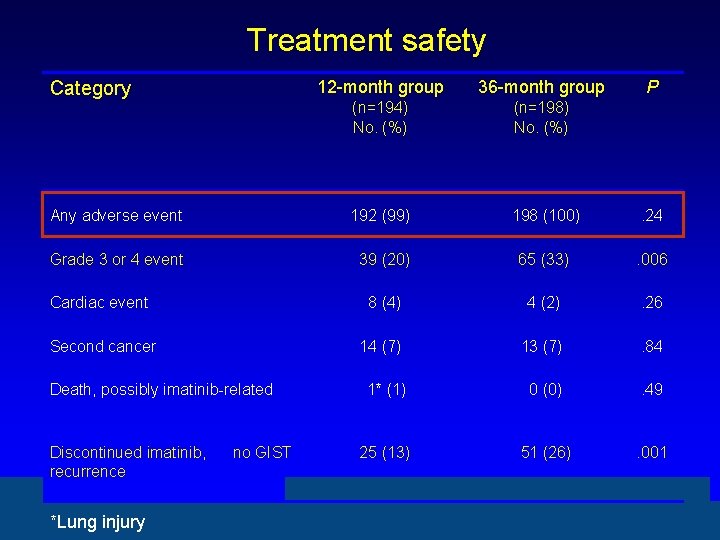
Treatment safety 12 -month group 36 -month group (n=194) No. (%) (n=198) No. (%) Any adverse event 192 (99) 198 (100) . 24 Grade 3 or 4 event 39 (20) 65 (33) . 006 Cardiac event 8 (4) 4 (2) . 26 Second cancer 14 (7) 13 (7) . 84 1* (1) 0 (0) . 49 25 (13) 51 (26) . 001 Category Death, possibly imatinib-related Discontinued imatinib, recurrence *Lung injury no GIST P

Most frequent adverse events Adverse event Any Grade 12 Mo P % 36 Mo % Grade 3 or 4 12 Mo P % 36 Mo % Anemia Periorbital edema 72 59 80 74 . 08. 002 1 1 1. 00 Elevated LDH* 43 60 . 001 0 0 - Fatigue 48 48 1. 00 1 1 . 62 Nausea 45 51 . 23 2 1 . 37 Diarrhea 44 54 . 044 1 2 . 37 Leukopenia 35 47 . 014 2 3 . 75 Muscle cramps 31 49 <0. 001 1 1 1. 00

Conclusions Ø Compared to 1 year of adjuvant imatinib, 3 years of imatinib improves - RFS - Overall survival as treatment of GIST patients who have a high estimated risk of recurrence after surgery. Ø Adjuvant imatinib is relatively well tolerated; severe adverse events are infrequent.

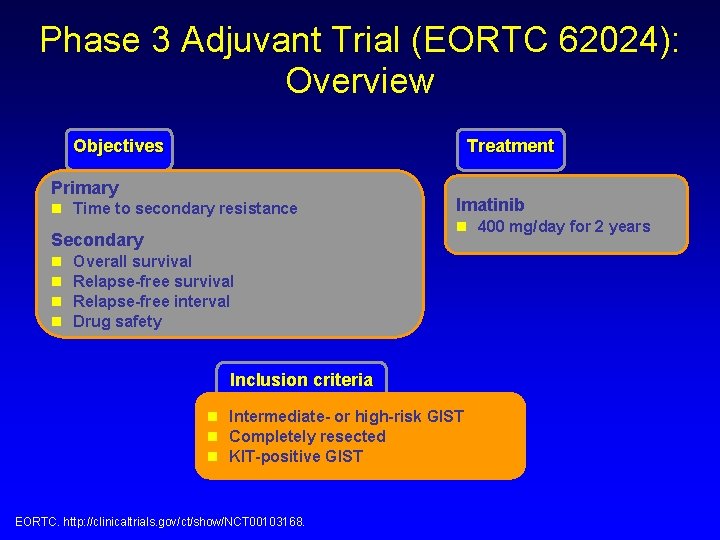
Phase 3 Adjuvant Trial (EORTC 62024): Overview Objectives Treatment Primary n Time to secondary resistance Secondary n n Imatinib n 400 mg/day for 2 years Overall survival Relapse-free interval Drug safety Inclusion criteria n Intermediate- or high-risk GIST n Completely resected n KIT-positive GIST EORTC. http: //clinicaltrials. gov/ct/show/NCT 00103168.

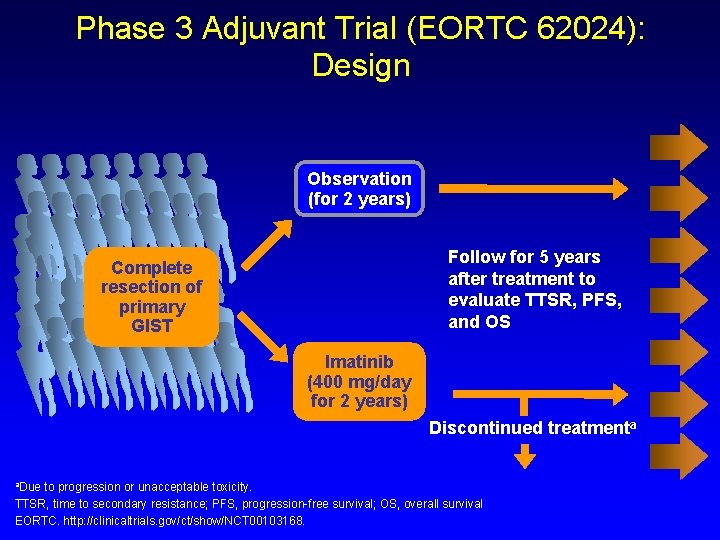
Phase 3 Adjuvant Trial (EORTC 62024): Design Observation (for 2 years) Follow for 5 years after treatment to evaluate TTSR, PFS, and OS Complete resection of primary GIST Imatinib (400 mg/day for 2 years) Discontinued treatmenta a. Due to progression or unacceptable toxicity. TTSR, time to secondary resistance; PFS, progression-free survival; OS, overall survival EORTC. http: //clinicaltrials. gov/ct/show/NCT 00103168.

Post-Resection Evaluation of Recurrence-Free Survival for Gastro-Intestinal Stromal Tumors Treated with Adjuvant Imatinib: PERSIST-5 Resected GIST >2 cm and mitotic rate >5 or Non-gastric primary >5 cm Register Imatinib 400 mg/d x 5 years Phase II N = 85 patients Primary objective: Recurrence-free survival Adapted De. Matteo

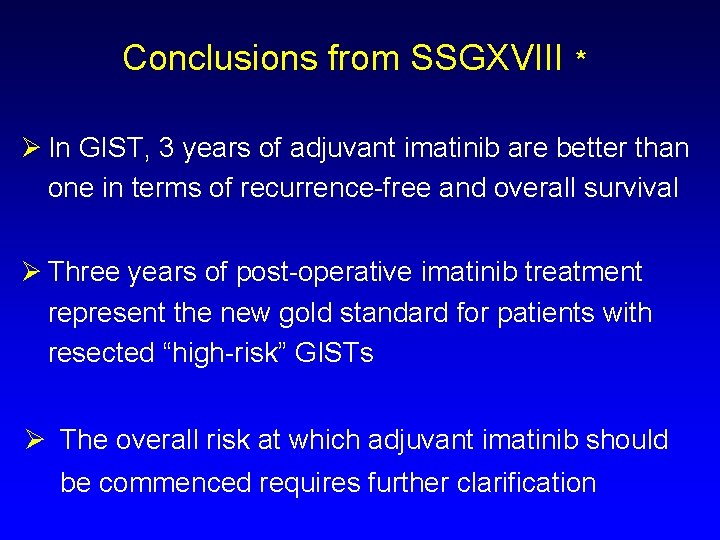
Conclusions from SSGXVIII * Ø In GIST, 3 years of adjuvant imatinib are better than one in terms of recurrence-free and overall survival Ø Three years of post-operative imatinib treatment represent the new gold standard for patients with resected “high-risk” GISTs Ø The overall risk at which adjuvant imatinib should be commenced requires further clarification
 Boundaries meme
Boundaries meme Short short short long long long short short short
Short short short long long long short short short Once upon a time there was a poor girl
Once upon a time there was a poor girl Tinikling props
Tinikling props Long long ago when the gods and goddesses
Long long ago when the gods and goddesses Chasing lincoln's killer map
Chasing lincoln's killer map Physical appearance of the weird sisters in macbeth
Physical appearance of the weird sisters in macbeth With whom did shilpi and her husband discuss pregnancy?
With whom did shilpi and her husband discuss pregnancy? Whose and whom
Whose and whom What does curley wear to set himself
What does curley wear to set himself Long time ago people
Long time ago people Once upon a time there lived a princess
Once upon a time there lived a princess Once upon a time a long long time ago
Once upon a time a long long time ago Long long int c
Long long int c Bọn em hai đứa cùng tên
Bọn em hai đứa cùng tên Once upon a time a long long time ago begins the story
Once upon a time a long long time ago begins the story Mình tròn thân trắng dáng hình thon thon
Mình tròn thân trắng dáng hình thon thon Who is mr. kirwin and how does he treat victor?
Who is mr. kirwin and how does he treat victor? We help our friends
We help our friends Consuelo ortiga y rey
Consuelo ortiga y rey Those he foreknew he also predestined
Those he foreknew he also predestined Brian camley
Brian camley Adjective clause who, whom, whose, which
Adjective clause who, whom, whose, which Relative pronoun練習
Relative pronoun練習 Whom shall i fear
Whom shall i fear Pims online reports
Pims online reports Who says what to whom in which channel with what effect
Who says what to whom in which channel with what effect From who did hanuman learn sacred astrology
From who did hanuman learn sacred astrology Why does snowball want to build a windmill
Why does snowball want to build a windmill Level 3 questions for cinderella
Level 3 questions for cinderella With whom should an na use standard precautions?
With whom should an na use standard precautions? Isaiah whom shall i send
Isaiah whom shall i send Whom i have in heaven but you
Whom i have in heaven but you By whoever or by whomever
By whoever or by whomever Joy in serving god
Joy in serving god Why does montresor feel sick at the end of the story
Why does montresor feel sick at the end of the story To he whom much is given
To he whom much is given