WHO Good Governance for Medicines programme WHO Technical
















- Slides: 23

WHO Good Governance for Medicines programme WHO Technical Briefing Seminar Dr Guitelle Baghdadi-Sabeti & Dr Sana Naffa 19 November 2008, Geneva Department Essential Medicines and Pharmaceutical Policies

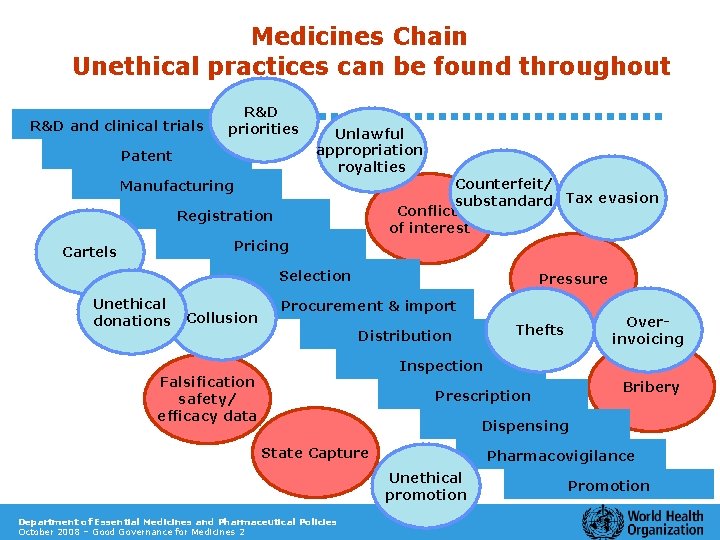
Medicines Chain Unethical practices can be found throughout R&D and clinical trials R&D priorities Patent Unlawful appropriation royalties Counterfeit/ substandard Tax evasion Conflict of interest Manufacturing Registration Pricing Cartels Selection Unethical donations Collusion Pressure Procurement & import Overinvoicing Thefts Distribution Inspection Falsification safety/ efficacy data Bribery Prescription Dispensing State Capture Pharmacovigilance Unethical promotion Department of Essential Medicines and Pharmaceutical Policies October 2008 – Good Governance for Medicines 2 Promotion

Corruption identified as the single greatest obstacle to economic and social development q US$ 4. 4 trillion spent on health services annually q Global pharmaceutical market: > US$ 600 b q 10 to 25% procurement spending lost into corruption (including health sector) q Some countries report that 2/3 medicines supplies lost through corruption and fraud in hospitals q Low quality trials exaggerate the benefits of treatment by an average of 34% q Bribery of high officials in regulatory authorities has led to unsafe medicines circulating on the market resulting in deaths Department of Essential Medicines and Pharmaceutical Policies October 2008 – Good Governance for Medicines 3

Unethical practices can have significant impact on health systems q q Health impact ì Unsafe medicines on the market ì Lack EM in health facilities ì Irrational use of medicines Economical impact ì ì q Pharma. expenditure low-income countries: § 10 -40% of public health budget § 20 -50% of total health care expenditures Poor most affected inequalities Image and trust impact ì Reduces government capacity ì Reduces credibility of health profession ì Erodes public trust Department of Essential Medicines and Pharmaceutical Policies October 2008 – Good Governance for Medicines 4

Corruption requires two parties: the corrupter and the corruptee "Whose is the greater blame? She who sins for pay or he who pays for sin? " Sor Juana Inés de la Cruz Department of Essential Medicines and Pharmaceutical Policies October 2008 – Good Governance for Medicines 5

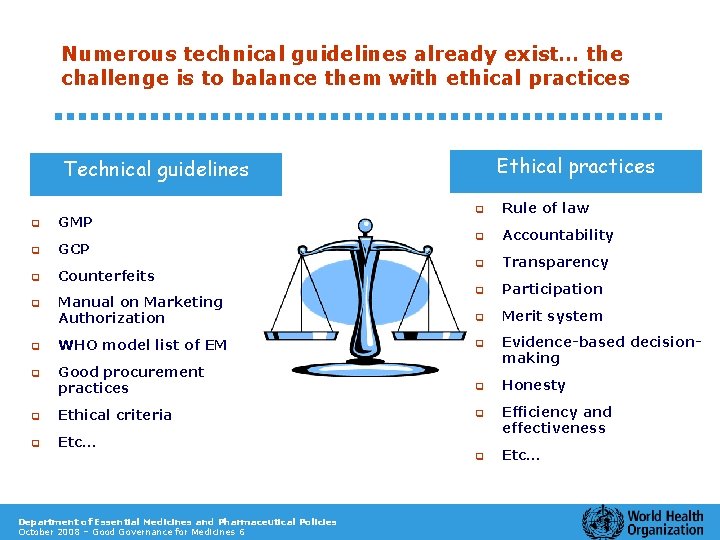
Numerous technical guidelines already exist… the challenge is to balance them with ethical practices Ethical practices Technical guidelines q q GMP GCP q Counterfeits q Manual on Marketing Authorization q Rule of law q Accountability q Transparency q Participation q Merit system q WHO model list of EM q q Good procurement practices Evidence-based decisionmaking q Honesty q Ethical criteria q q Etc… Efficiency and effectiveness q Etc… Department of Essential Medicines and Pharmaceutical Policies October 2008 – Good Governance for Medicines 6

WHO Good Governance for Medicines Programme q Goal ì q To curb corruption in pharmaceutical sector systems through the application of transparent and accountable administrative procedures and the promotion of ethical practices among health professionals. Specific objectives ì To increase the awareness of all stakeholders on the potential for corruption in the pharmaceutical sector and its impact on health systems functioning. ì To increase transparency and accountability in medicines regulatory systems and supply management systems. ì To build national capacity for good governance in medicines regulation and supply management systems. Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 7

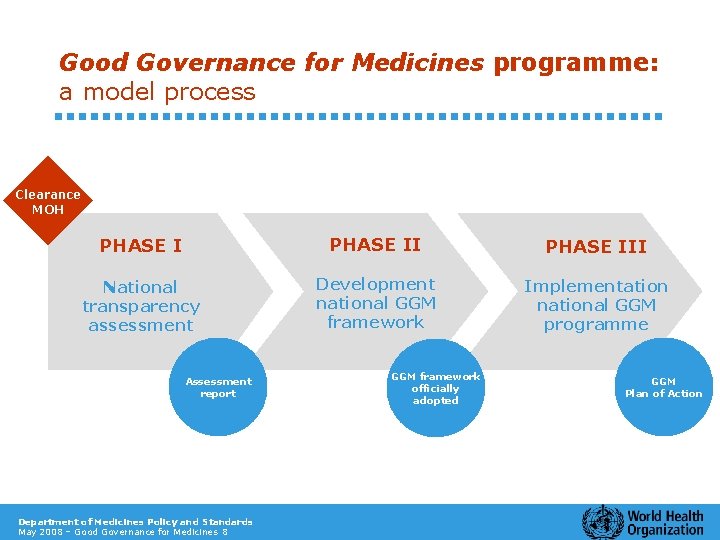
Good Governance for Medicines programme: a model process Clearance MOH PHASE III National transparency assessment Development national GGM framework Implementation national GGM programme Assessment report Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 8 GGM framework officially adopted GGM Plan of Action

Efforts to address corruption need coordinated application of two basic strategies q "Discipline-based approach" (top-down) ì ì q Laws, policies and procedures against corruption and for pharmacy practice with adequate punitive consequence for violation Attempts to prevent corrupt practices through fear of punishment "Values-based approach" (bottom-up) ì ì Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 9 Promotes institutional integrity through promotion moral values and ethical principles Attempts to motivate ethical conduct of public servant

What could be the components of a national GGM Framework? 1. Ethical framework of moral values & ethical principles ì Justice/fairness ì Truth ì Service to common good ì trusteeship 2. Code of conduct 3. Socialization programme 4. Promotion of Moral Leadership Values based approach Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 10 5. Established anti-corruption legislation 6. Whistle-blowing mechanism 7. Sanctions on reprehensible acts 8. Transparent and accountable regulations and administrative procedures 9. Collaboration with other GG & AC initiatives 10. Management, coordination and evaluation of GGM programme (Steering Committee & task force) Discipline based approach

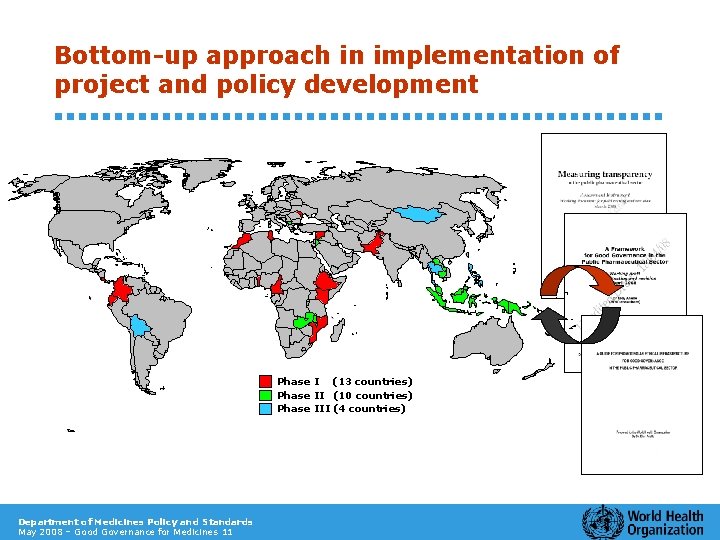
Bottom-up approach in implementation of project and policy development Phase I (13 countries) Phase II (10 countries) Phase III (4 countries) Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 11

GGM Experience in Jordan Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 12

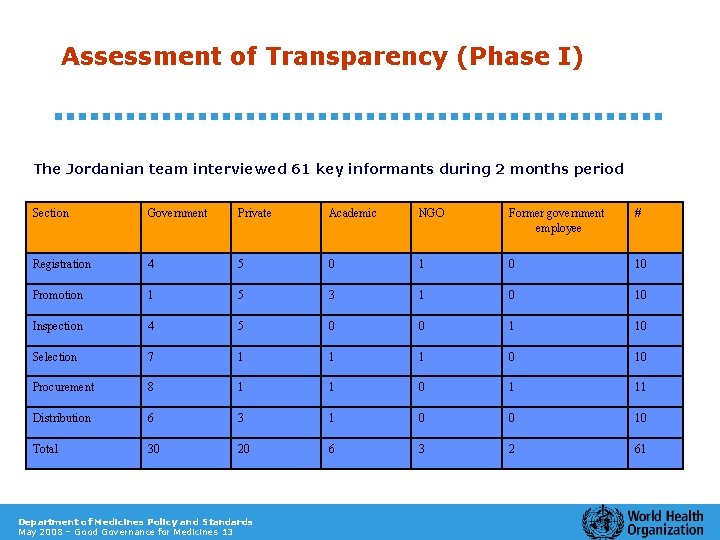
Assessment of Transparency (Phase I) The Jordanian team interviewed 61 key informants during 2 months period Section Government Private Academic NGO Former government employee # Registration 4 5 0 10 Promotion 1 5 3 1 0 10 Inspection 4 5 0 0 1 10 Selection 7 1 1 1 0 10 Procurement 8 1 1 0 1 11 Distribution 6 3 1 0 0 10 Total 30 20 6 3 2 61 Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 13

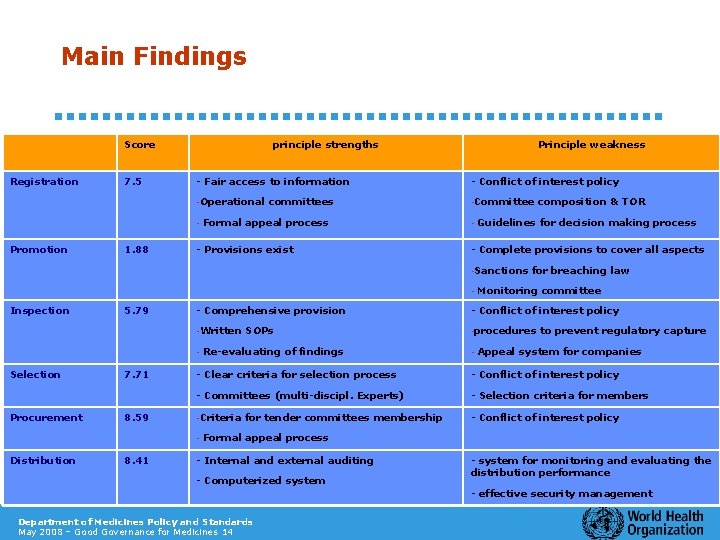
Main Findings Score Registration 7. 5 principle strengths - Fair access to information - Conflict of interest policy -Operational -Committee - Promotion 1. 88 Principle weakness committees Formal appeal process - Provisions exist - Guidelines for decision making process - Complete provisions to cover all aspects -Sanctions - Inspection 5. 79 Procurement 7. 71 8. 59 8. 41 Monitoring committee - Conflict of interest policy -Written -procedures SOPs Re-evaluating of findings - to prevent regulatory capture Appeal system for companies - Clear criteria for selection process - Conflict of interest policy - Committees (multi-discipl. Experts) - Selection criteria for members -Criteria - Conflict of interest policy - Distribution for breaching law - Comprehensive provision - Selection composition & TOR for tender committees membership Formal appeal process - Internal and external auditing - Computerized system - system for monitoring and evaluating the distribution performance - effective security management Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 14

National GGM Workshop q The meeting targeted national stakeholders sectors q The objectives were: ì To introduce and raise awareness on the concept of GGM program ì To Disseminate GGM assessment results ì To develop recommendations on strengthening the systems ì To provide advice for members of the steering committee and task force ì To Broaden the vision for possible obstacles that may be faced in phase II, and the way that can be addressed Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 15

National Workshop Recommendations q To publish the national assessment report and disseminate it for all stakeholders and KI’s q To start doing self evaluation for their performance, and SWOT analysis in each department in the public sector. q To develop and adopt National Framework for Good Governance in the public pharmaceutical sector q To raise awareness in public services on the concept of governance Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 16

National Workshop follow up q Preparing a report of the meeting and sent to the minister of health; q Getting the official approval from the minister of health for GGM phase II activities was granted; q Preparing the TOR for GGM Steering Committee (SC) and Task Force (TF); q Nominating and assigning members of the SC formally by a minister decree; q Finalizing the assessment report; q Selecting and getting formal approvals on members of the TF who are working now on the development of the GGM ethical infrastructure; q Addressing and improving some of the principle weaknesses of the assessment report. Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 17

GGM Task Force q Manage the national consultations process necessary to: ì Share the results of the national transparency assessments ì Develop the national GGM framework ì Implement the national GGM programme q Follow up and act upon the recommendations made in the transparency assessments report measuring q Coordinate the development and adoption of the national GGM framework for promoting good governance in the public pharmaceutical sector q Coordinate the development and adoption of the code of conduct q Socialize the national ethical framework and the code of conduct Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 18

GGM Steering Committee q Ensure implementation of recommendations made in assessment report q Ensure that the national Framework for Good Governance in the public pharmaceutical sector is officially adopted q Ensure the establishment of policies and procedures for the control of reprehensible acts q Ensure the establishment of a whistle-blowing mechanism q Ensures the establishment of a GGM programme and GGM implementing Task Force (phase III) Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 19

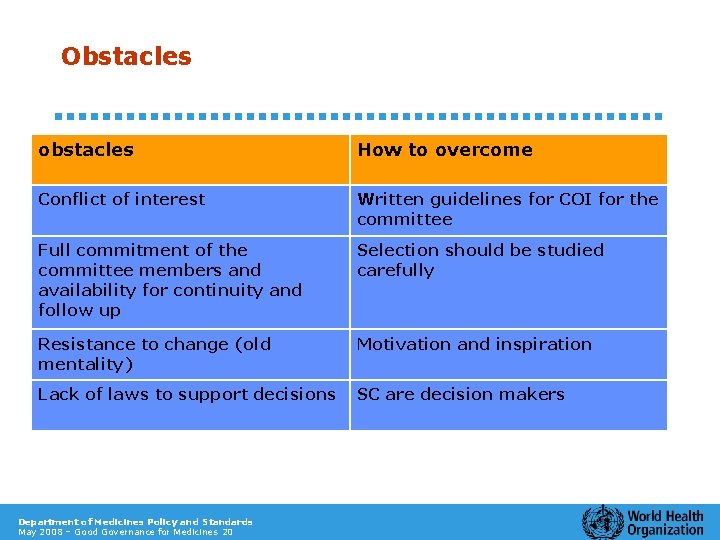
Obstacles obstacles How to overcome Conflict of interest Written guidelines for COI for the committee Full commitment of the committee members and availability for continuity and follow up Selection should be studied carefully Resistance to change (old mentality) Motivation and inspiration Lack of laws to support decisions SC are decision makers Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 20

Lessons Learnt to date q The foundation of ethical practices to strengthen governance in the pharmaceutical sector requires the creation of an effective integrity system; q The current culture and practices are important to be addressed when formulating the ethical framework; q Stakeholders are interested and keen to work in this area q Involvement of high-level policy makers is important q Task force committee work is needed for both the mid- term as well as for long-term strategies, the committee should be permanent and institutionalized; q A small group of motivated committed technical people can initiate the process and make a difference q There is still much more to do… Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 21

"Corruption is a powerful force, but it is not inevitable or unavoidable. Diminishing its impact restores diverted resources to their intended purpose, bringing better health, nutrition and education to victims of corruption around the world, and with them, opportunity and hope. " Transparency International Department of Medicines Policy and Standards May 2008 – 2007 September Good–Governance Good Governance for Medicines 22 22

Thank you for listening Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 23