WHO Good Governance for Medicines programme Me TA












- Slides: 15

WHO Good Governance for Medicines programme Me. TA Launch Dr Guitelle Baghdadi-Sabeti Geneva, 21 May 2008 st 61 World Health Assembly Department of Medicines Policy and Standards

Corruption identified as the single greatest obstacle to economic and social development q US$ 3 trillion spent on health services annually q Global pharmaceutical market: > US$ 600 b q 10 to 25% procurement spending lost into corruption (including health sector) q Some countries report that 2/3 medicines supplies lost through corruption and fraud in hospitals q Low quality trials exaggerate the benefits of treatment by an average of 34% q Bribery of high officials in regulatory authorities has led to unsafe medicines circulating on the market resulting in deaths Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 2

Unethical practices can be found throughout medicines chain & are very diverse R&D and clinical trials Evergreening R&D priorities Collusion Patent Fraud Manufacturing Overinvoicing Registration Falsification of safety/Efficacy data Thefts Cartels Pricing Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 3 Unethical donations Procurement & import Unethical promotion Pressure Counterfeit/ substandards Selection Conflict of interest State/regulatory capture Bribery Tax evasion Distribution Promotion Inspection

Unethical practices can have significant impact on health systems q Health impact ì Unsafe medicines on the market ì Lack EM in health facilities ì Irrational use of medicines q Economical impact ì Pharma. expenditure low-income countries: § ì 10 -40% of public health budget § 20 -50% of total health care expenditures Poor most affected inequalities q Image and trust impact ì Reduces government capacity ì Reduces credibility of health profession ì Erodes public trust Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 4

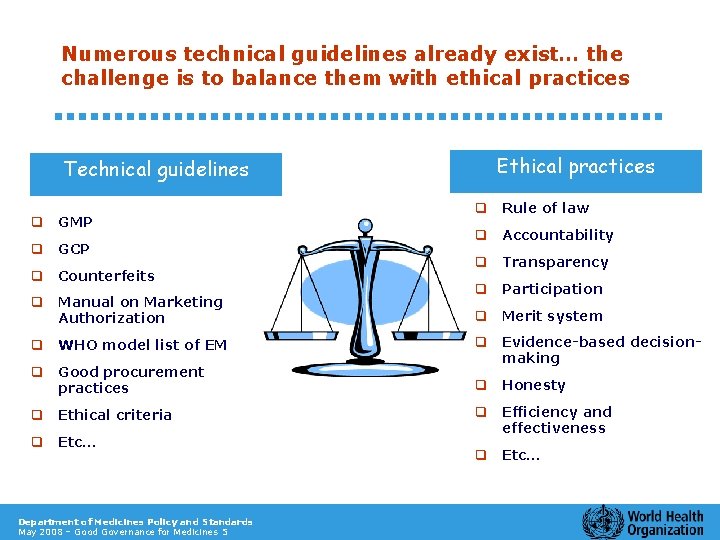
Numerous technical guidelines already exist… the challenge is to balance them with ethical practices Technical guidelines q GMP q GCP q Counterfeits q Manual on Marketing Authorization q WHO model list of EM q Good procurement practices q Ethical criteria q Etc… Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 5 Ethical practices q Rule of law q Accountability q Transparency q Participation q Merit system q Evidence-based decisionmaking q Honesty q Efficiency and effectiveness q Etc…

WHO Good Governance for Medicines Programme q Goal ì To curb corruption in pharmaceutical sector systems through the application of transparent and accountable administrative procedures and the promotion of ethical practices among health professionals. q Specific objectives ì To increase the awareness of all stakeholders on the potential for corruption in the pharmaceutical sector and its impact on health systems functioning. ì To increase transparency and accountability in medicines regulatory systems and supply management systems. ì To build national capacity for good governance in medicines regulation and supply management systems. Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 6

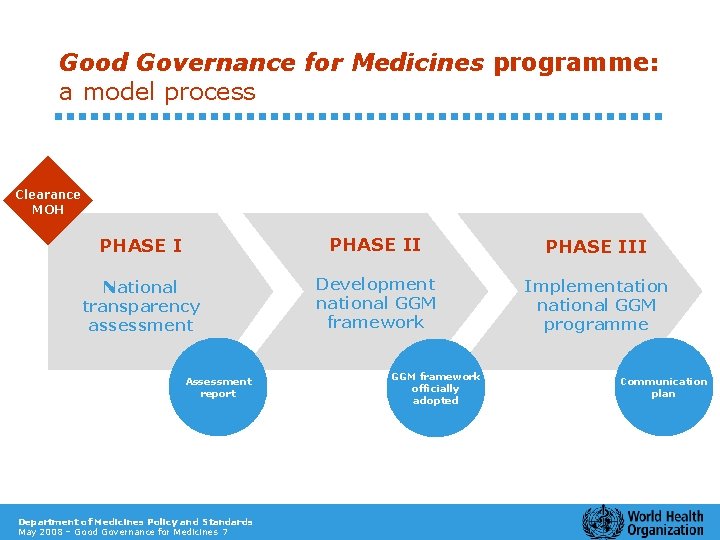
Good Governance for Medicines programme: a model process Clearance MOH PHASE III National transparency assessment Development national GGM framework Implementation national GGM programme Assessment report Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 7 GGM framework officially adopted Communication plan

Efforts to address corruption need coordinated application of two basic strategies q "Discipline-based approach" (top-down) ì Laws, policies and procedures against corruption and for pharmacy practice with adequate punitive consequence for violation ì Attempts to prevent corrupt practices through fear of punishment q "Values-based approach" (bottom-up) ì Promotes institutional integrity through promotion moral values and ethical principles ì Attempts to motivate ethical conduct of public servant Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 8

What could be the components of a national GGM Framework? 1. Ethical framework of moral values & ethical principles 5. Established anti-corruption legislation ì Justice/fairness 6. Whistle-blowing mechanism ì Truth 7. Sanctions on reprehensible acts ì Service to common good ì trusteeship 2. Code of conduct 3. Socialization programme 4. Promotion of Moral Leadership Values based approach Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 9 8. Transparent and accountable regulations and administrative procedures 9. Collaboration with other GG & AC initiatives 10. Management, coordination and evaluation of GGM programme (Steering Committee & task force) Discipline based approach

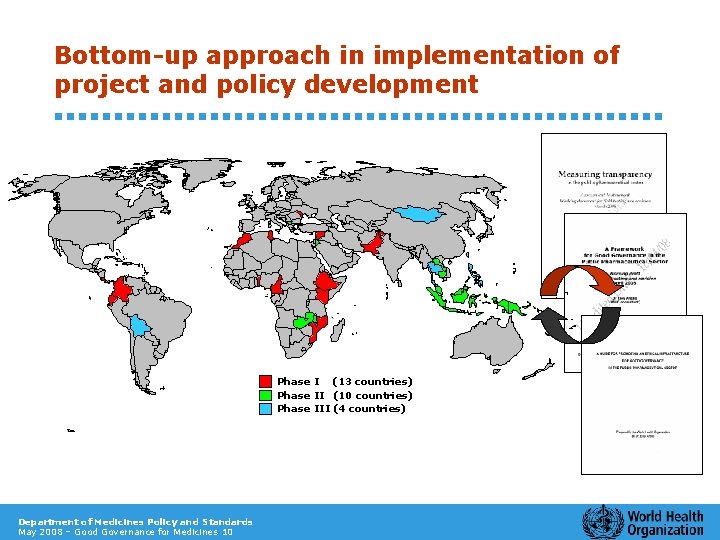
Bottom-up approach in implementation of project and policy development Phase I (13 countries) Phase II (10 countries) Phase III (4 countries) Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 10

PHASE III Progress in countries q Nb countries: ì 18 completed ì 9 currently on-going q Publications: ì 4 -country study: Laos, Malaysia, Philippines, Thailand ì 5 -country study: Bolivia, Cambodia, PNG, Mongolia, Indonesia (upcoming) ì Future: individual country reports Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 11

PHASE III Progress in countries q National workshops: ì Share results assessment ì Consult on national GGM framework q National GGM Steering Group and/or Task Force q Consultation phase to finalize national GGM framework q Official adoption of national GGM framework Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 12

PHASE III Progress in countries q Mongolia ì Official establishment of national GGM committee ì Regional technical groups (including training) ì Campaigns to promote awareness (educational material) q Philippines ì GGM pharmaceutical benchbook ì Awards system for local units q Thailand ì Workshops on GGM framework ì Newsletters, public communications (media, brochures, websites) ì Introduction in university curricula q Bolivia (waiting clearance Po. A by MOH) ì Develop national GGM programme (national and regional consultations) ì Orientation meeting for MOH staff ì Campaign for promoting awareness Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 13

Key next steps for 2008 q Analyse experience from 4 phase III countries and further refine WHO global guidance q Establish system to collect learning in countries and facilitate communications b/w countries q Scale up to more countries q Publish more country assessment reports q Next Global Stakeholders Group in Alexandria q Explore collaboration with private sector q Raise funding for wider implementation of the programme Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 14

"I never worry about action, but only inaction. " Winston CHURCHILL Department of Medicines Policy and Standards May 2008 – Good Governance for Medicines 15
 Refrigerant management program
Refrigerant management program Buenas tardes good afternoon
Buenas tardes good afternoon You are good when theres nothing good in me
You are good when theres nothing good in me Good thoughts good deeds good actions
Good thoughts good deeds good actions Good evening students
Good evening students Good morning
Good morning Niyog niyogan health benefits
Niyog niyogan health benefits National medicines policy
National medicines policy Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Stockley’s drug interaction
Stockley’s drug interaction Glencoe health chapter 19 medicines and drugs
Glencoe health chapter 19 medicines and drugs Medicines information centre
Medicines information centre European directorate for the quality of medicines
European directorate for the quality of medicines Ggc medicines
Ggc medicines European medicines agency
European medicines agency Staff of marvelous medicines
Staff of marvelous medicines