WHO Global Regulatory Model for medical devices Justification

WHO Global Regulatory Model for medical devices

• • • Justification and target audience Key concepts in regulating medical devices How to start regulating medical devices Challenges for low and middle income countries How to tackle those challenges

WHO mandate WHA 67. 20 Regulatory System Strengthening for medical products … to prioritize support for establishing and strengthening regional and subregional networks of regulatory authorities, as appropriate, including strengthening areas of regulation of health products that are the least developed, such as regulation of medical devices, including diagnostics; http: //apps. who. int/gb/ebwha/pdf_files/WHA 67/A 67_R 20 -en. pdf
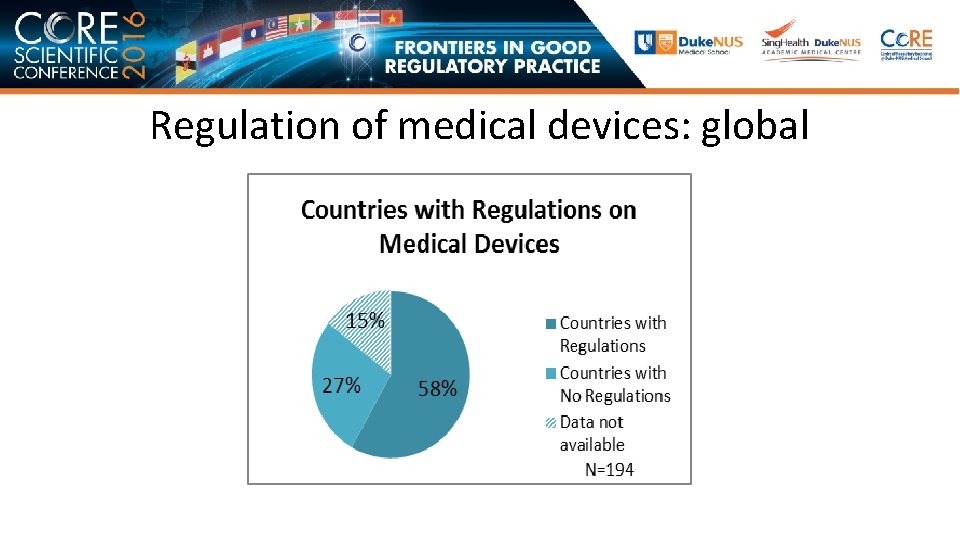
Regulation of medical devices: global
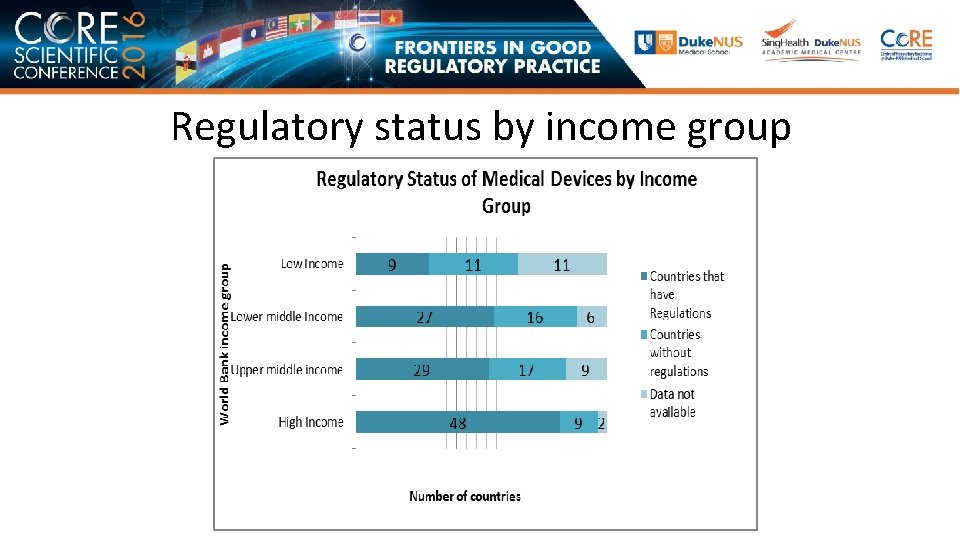
Regulatory status by income group


Target audience • Countries with no or limited regulatory framework in place • With the ambition to improve this situation • The legislative, executive and regulatory branches of government

Convergence and harmonization • • Definition of a medical device Classification of medical devices Essential principles of safety and performance QMS Standards … • Reliance and recognition

Two step approach Basic level controls and enforcement • Legal framework • Market oversight • Reporting system Expanded level controls and enforcement • regulatory controls depending on the priorities of the country


Regulating medical devices: challenges • Less developed regulatory systems than for vaccines and medicines, particularly in LIMC’s • Lack of awareness • Characteristics of medical devices as a product group • Regulating in an existing market • Lack of specialized knowledge and resources to draft and implement medical devices regulations • Lack of resources

Critical elements for regulating medical devices Political commitment Legal framework Implementation plan including gap analysis Monitoring implementation Competent authority with enforcement power Involvement of stakeholders Transparent and impartial

WHO Global Model Regulatory Framework: the ambition Enables a harmonized regulatory environment Allows for better protection of public health Facilitates the availability of medical technologies consistent with requirement of safety and performance Provides a clear system of regulatory controls Use of harmonized, coordinated controls enabling cross-border leveraging of regulatory resources and reduces burdens to industry

- Slides: 14