WHO Collaborating Centre for International Drug Monitoring The

WHO Collaborating Centre for International Drug Monitoring The WHO Programme for International Drug Monitoring The Uppsala Monitoring Centre Monica Plöen

WHO Collaborating Centre the Uppsala Monitoring Centre • Established as a foundation 1978 • Based on agreement Sweden - WHO • International administrative board • WHO Headquarters responsible for policy • Self financing

UMC activities WHO Programme Funding Commercial sector activities WHO Drug Dictionaries
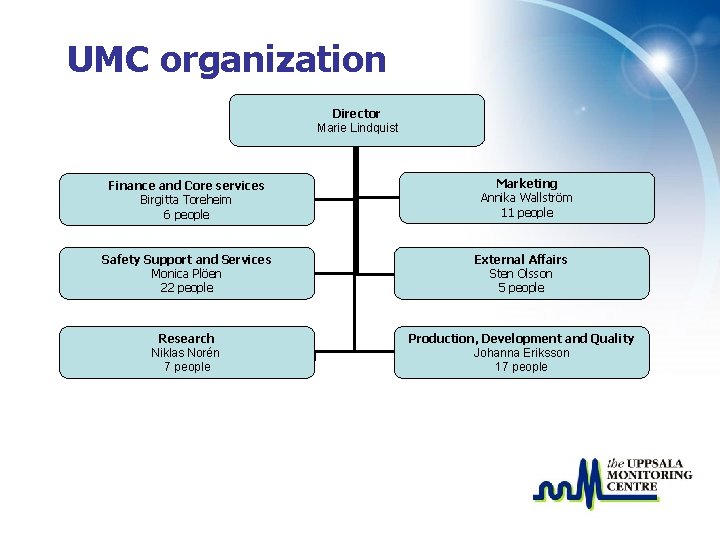
UMC organization Director Marie Lindquist Finance and Core services Birgitta Toreheim 6 people Marketing Annika Wallström 11 people Safety Support and Services Monica Plöen 22 people External Affairs Sten Olsson 5 people Research Niklas Norén 7 people Production, Development and Quality Johanna Eriksson 17 people
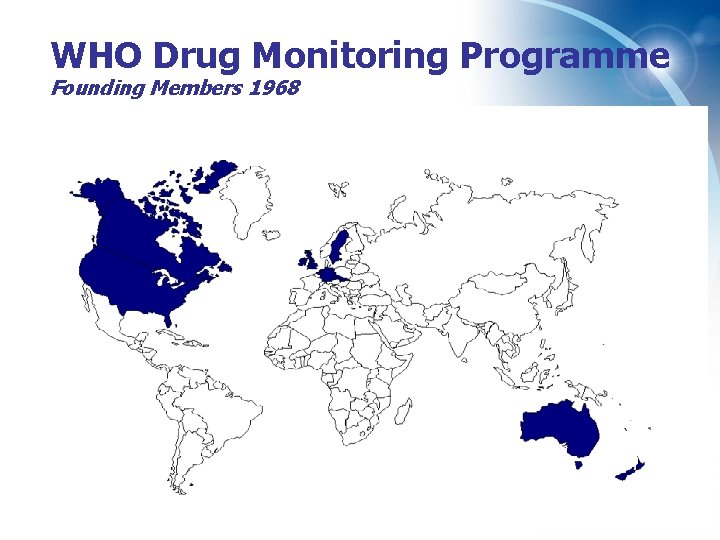
WHO Drug Monitoring Programme Founding Members 1968
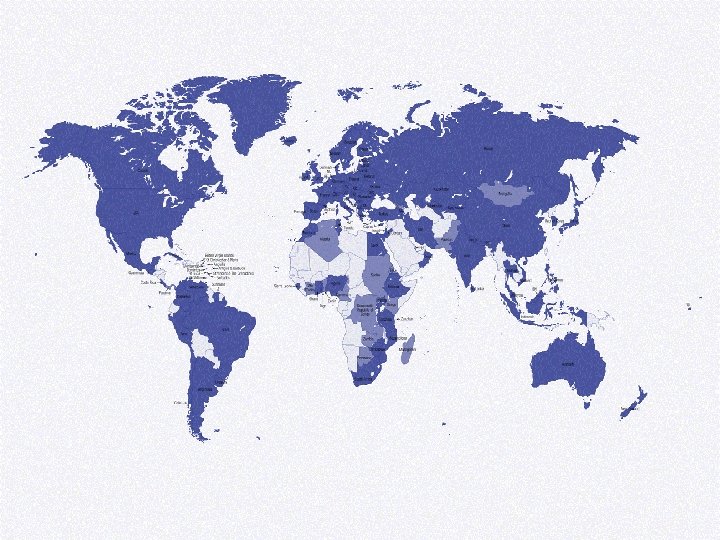
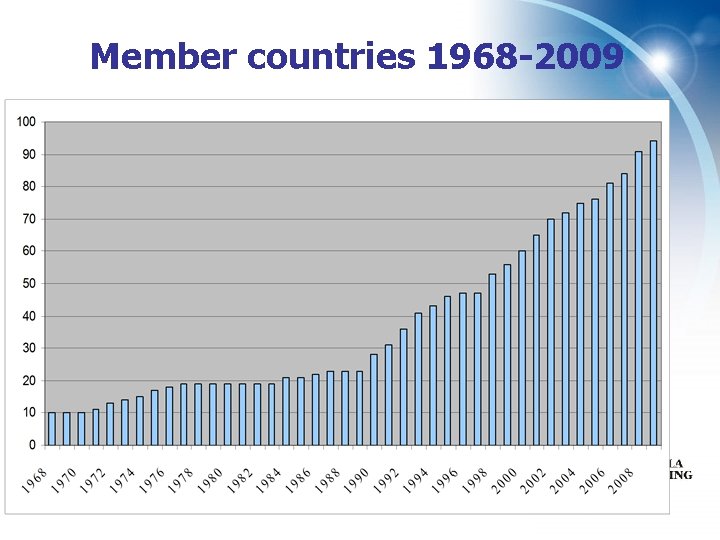
Member countries 1968 -2009
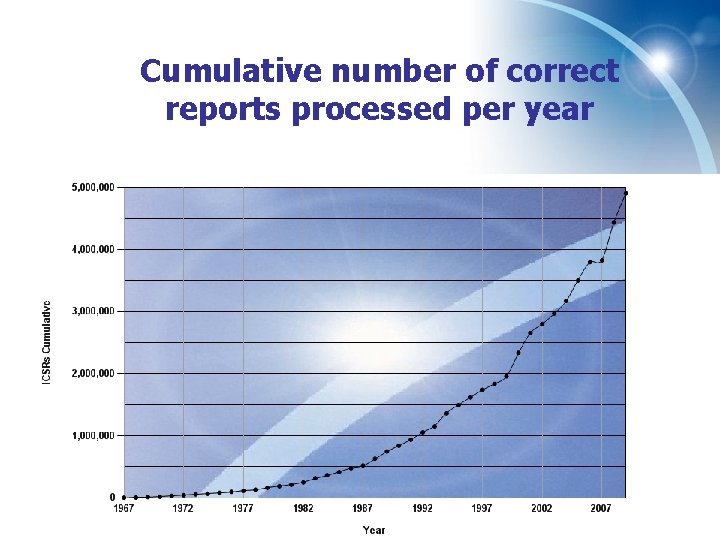
Cumulative number of correct reports processed per year
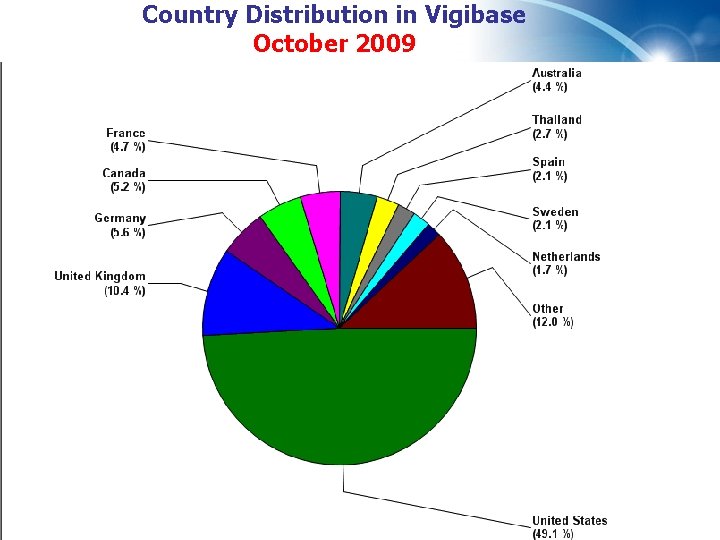
Country Distribution in Vigibase October 2009

Vigi. Flow – a software for management of ADR case data • Swiss. Medic 2001 • Free text possible • Web based • Mandatory fields • E 2 B format • Error checks • Less report delay • National database
UMC Function 1 Signal detection • Primary UMC task • Identification of previously unknown drug reactions
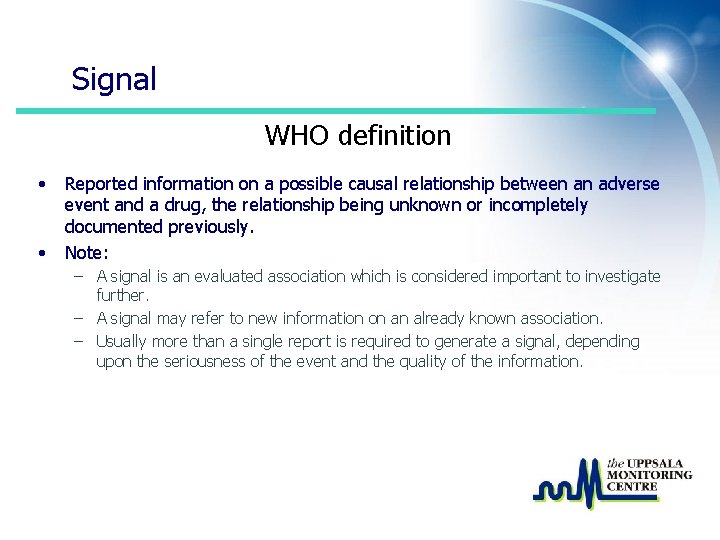
Signal WHO definition • • Reported information on a possible causal relationship between an adverse event and a drug, the relationship being unknown or incompletely documented previously. Note: – A signal is an evaluated association which is considered important to investigate further. – A signal may refer to new information on an already known association. – Usually more than a single report is required to generate a signal, depending upon the seriousness of the event and the quality of the information.
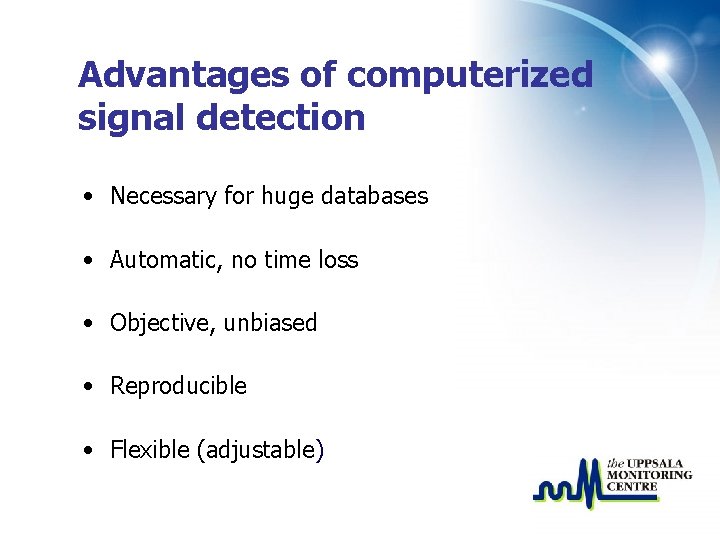
Advantages of computerized signal detection • Necessary for huge databases • Automatic, no time loss • Objective, unbiased • Reproducible • Flexible (adjustable)
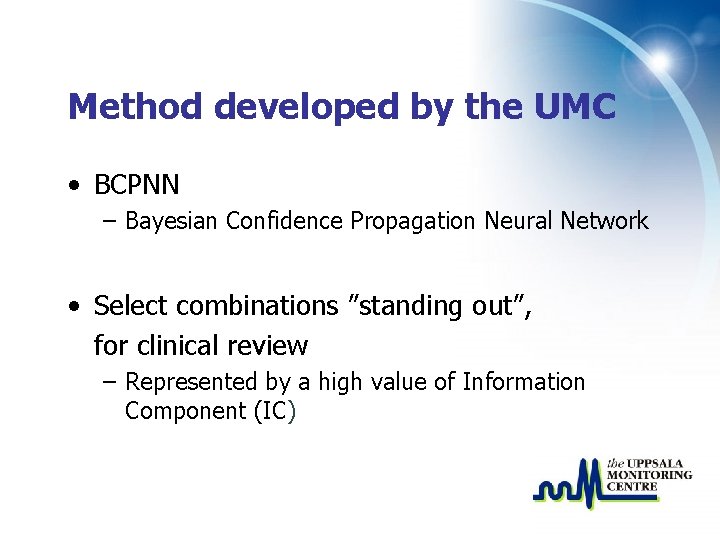
Method developed by the UMC • BCPNN – Bayesian Confidence Propagation Neural Network • Select combinations ”standing out”, for clinical review – Represented by a high value of Information Component (IC)
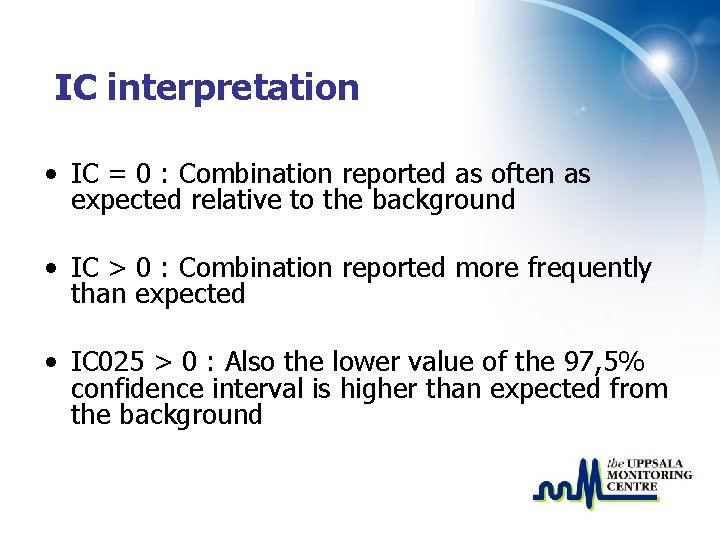
IC interpretation • IC = 0 : Combination reported as often as expected relative to the background • IC > 0 : Combination reported more frequently than expected • IC 025 > 0 : Also the lower value of the 97, 5% confidence interval is higher than expected from the background
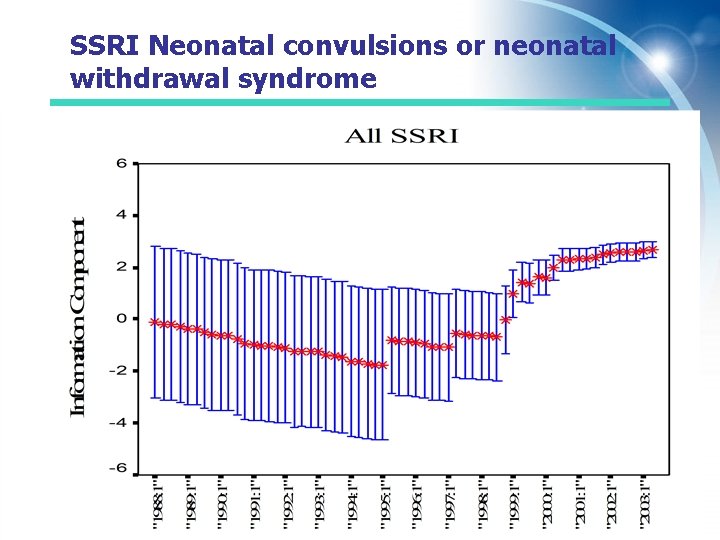
SSRI Neonatal convulsions or neonatal withdrawal syndrome
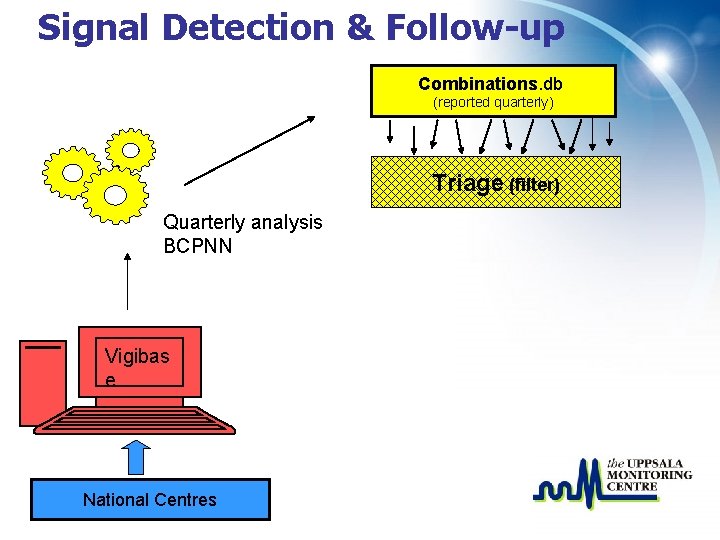
Signal Detection & Follow-up Combinations. db (reported quarterly) Triage (filter) Quarterly analysis BCPNN Vigibas e National Centres
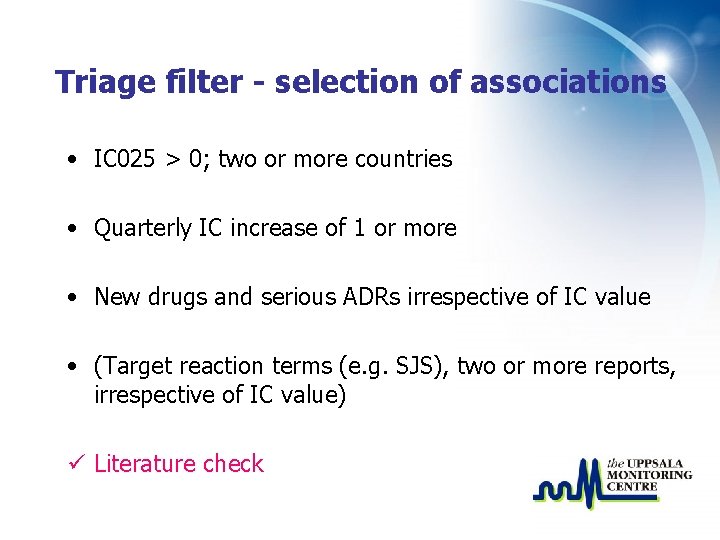
Triage filter - selection of associations • IC 025 > 0; two or more countries • Quarterly IC increase of 1 or more • New drugs and serious ADRs irrespective of IC value • (Target reaction terms (e. g. SJS), two or more reports, irrespective of IC value) ü Literature check
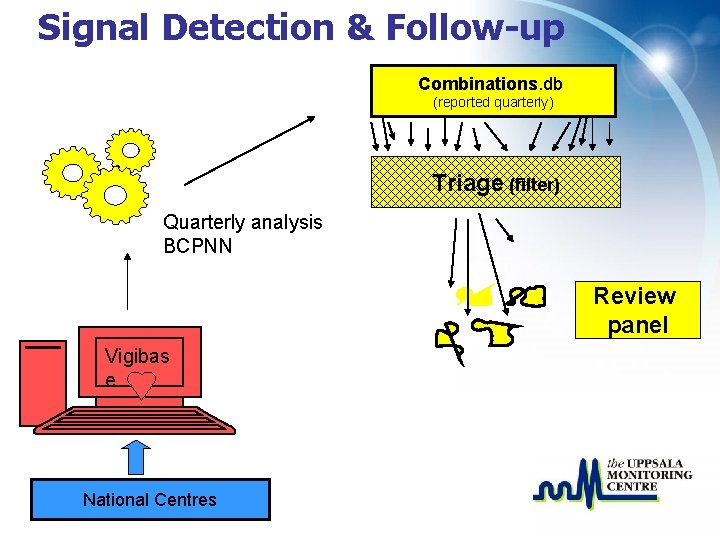
Signal Detection & Follow-up Combinations. db (reported quarterly) Triage (filter) Quarterly analysis BCPNN Review panel Vigibas e National Centres

Signal review panel • 40 experts from around the world • Evaluate signals, together with UMC staff and National Centres • Select associations for follow-up • Write signals in the SIGNAL document

The SIGNAL document • Sent to all National Centres • Individualized section available to industry • All recipients encouraged to comment on topics presented Or published in WHO Pharmaceutical Newsletter
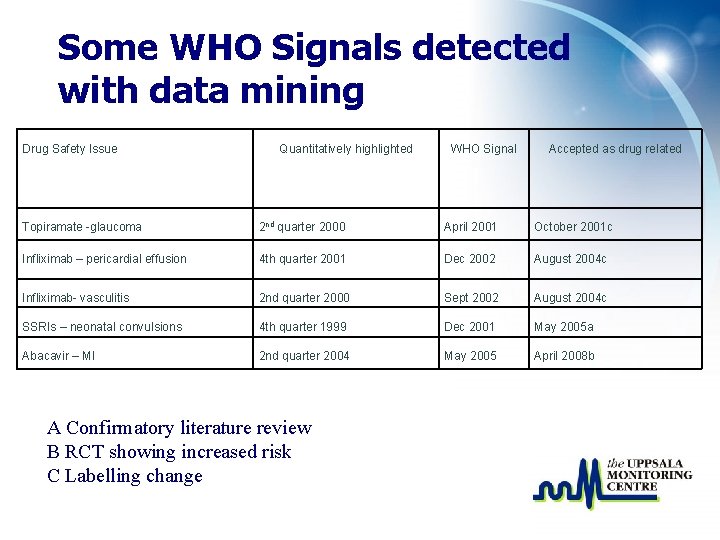
Some WHO Signals detected with data mining Drug Safety Issue Quantitatively highlighted WHO Signal Accepted as drug related Topiramate -glaucoma 2 nd quarter 2000 April 2001 October 2001 c Infliximab – pericardial effusion 4 th quarter 2001 Dec 2002 August 2004 c Infliximab- vasculitis 2 nd quarter 2000 Sept 2002 August 2004 c SSRIs – neonatal convulsions 4 th quarter 1999 Dec 2001 May 2005 a Abacavir – MI 2 nd quarter 2004 May 2005 April 2008 b A Confirmatory literature review B RCT showing increased risk C Labelling change

UMC functions 2 • Signal strengthening – Web-based search programme (Vigisearch/Vigimine) – Search requests

Data available to non-members • By request to WHO Collaborating Centre • To degree health professionals • Caveat document

UMC functions 3 • Comparing national experiences

International Differences (Quantitative and Qualitative) • • • Disease prevalence Genetic Social Cultural Healthcare systems Health professional practices Indication for, and use of medicines Pharmaceutical formulations Drug monitoring practices

UMC functions 4 • Identification of risk factors

Potential Risk Factors • • Other drugs Sex/gender Age Genetic constitution Dosage Duration of treatment Route of administration Indication

WHO Drug Dictionary • A source of international drug names • Includes all drugs reported to Vigi. Base • Information on MAH, form, strength, source etc. • Drugs classified according to the ATC (Anatomical. Therapeutic-Chemical) classification system • Ingredient names according to INN
WHO herbal ADR database Valid scientific botanical names • No internationally standardized and accepted classification of all botanical names of medicinal herbs exist • the UMC has created a list of preferred botanical names and their synonyms

The common name problem Common name Botanical name Chemical relation Chinese, Asian Ginseng American Ginseng Tienchi Ginseng Siberian Ginseng Russian Ginseng Brazilian Ginseng Wild red Am. Ginseng Alaskan Ginseng Wild Ginseng Ayurvedic Ginseng of the Andes’ Panax ginseng Meyer Standard Panax quinquefolius L. Similar Panax pseudoginseng Wall. Similar Eletherococcus senticosus Maxim. Different Acanthopanax senticosus Harms. Different Rumex hymenosepalus Torr. Different Pfaffia paniculata (Mart. ) Kunze. Different Echinopanax horridum (Sm. ) Decne. Different Aralia nudicaulis L. Different Withania somnifera (L. ) Dunal Different Lepidium meyenii Walpers Different





Technical support to the WHO Programme • Guidelines – Why and how to set up PV centres • Terminologies – WHO Adverse Reaction Terminology – WHO Drug Dictionary • Software development – – – Vigiflow Paniflow CEM-flow Vigisearch/Vigimine DD Browser


UMC involvement in local activities 2005 -2009 • 2005 – India, Germany, Moldova, Turkey, Italy, Poland, Argentina • 2006 – Uzbekistan, Brazil, Barbados • 2007 – India, Nepal, South Africa, Ghana, China, UAE • 2008 – Namibia, Philippines, India, Botswana • 2009 – Uganda, Saudi Arabia, India, Tanzania, Nigeria, Mozambique

UMC - a communication centre • WHO Pharmaceuticals Newsletter • Uppsala Reports • Internet home page http: //www. who-umc. org • Vigimed e-mail discussion group

Thank you for your attention!
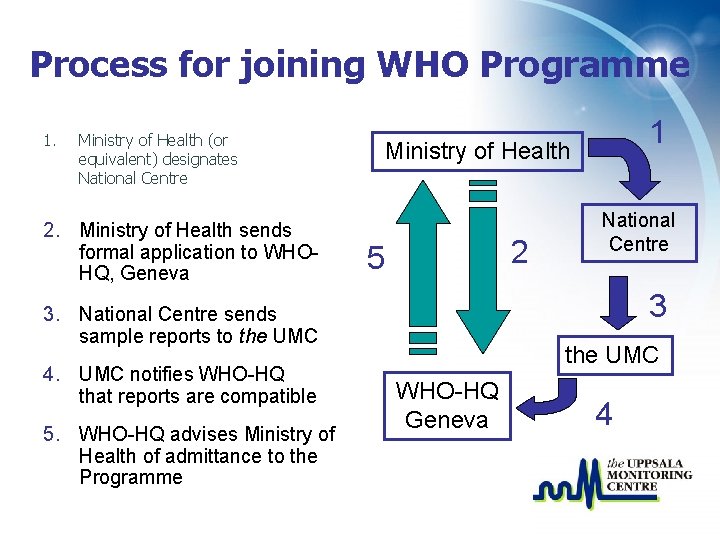
Process for joining WHO Programme 1. Ministry of Health (or equivalent) designates National Centre 2. Ministry of Health sends formal application to WHOHQ, Geneva Ministry of Health 2 5 5. WHO-HQ advises Ministry of Health of admittance to the Programme National Centre 3 3. National Centre sends sample reports to the UMC 4. UMC notifies WHO-HQ that reports are compatible 1 the UMC WHO-HQ Geneva 4
- Slides: 42