WHO Assessment of National Medicines Regulatory Authorities NMRAs















- Slides: 29

WHO Assessment of National Medicines Regulatory Authorities (NMRAs) WHO/UNICEF Technical Briefing Seminar on Essential Medicines Policies WHO Headquaters, Geneva, Switzerland 31 October - 4 November 2011 l Alain PRAT, Technical adviser, QSM/EMP/HSS

Content of the presentation l Assessment tool and process l Figures and findings l Future perspectives l References 2| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Why an assessment tool ? In line with one of the strategic objectives – To strengthen National Medicines Regulatory Authorities (NMRAs) capacities l To provide for evidence – on the situation by identifying strengths and weaknesses – on the improvement by comparison l To make recommendations on identified gaps for improvement l To use assessment results as a tool for convincing decisionmakers to gain support 3| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Main steps of the assesssment / 1 l Expression of a need – Internal / External – Scope of the assessment – Objectives and expected outcomes l Assessment team – Qualification, experience, availability – Minimun 2 – Staff from the organization assessed l Preparation works – – – 4| Request baseline information Study of available information Validation of the scope covered Preparation of the assessment plan Validation of the plan with the institution Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Main steps of the assessment / 2 l Opening session – Presentation of assessment team, objectives, methodology – Presentation of the authority l Conducting the visit – Follow planned activities, – Collection of evidence l Closing session – Presentation of the main findings and related recommendations – Presentation of the institutional plan – Closing remarks l Follow up – Provide for the draft report, collect the comments and finalize – Initiate/consider supportive actions 5| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

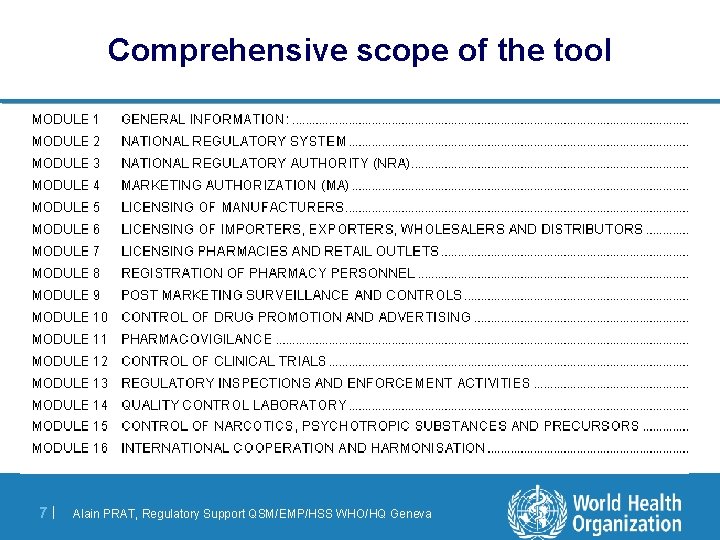
Design of the assessment tool l Same format for each modules / functions – – – – 6| Legal basis, framework Guideline and Documentation Organisation and structure Planning and internal procedures Human and other Ressources Records and others outputs Availability of these information Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Comprehensive scope of the tool 7| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Level of scrutiny - Granulometry Limited number of indicators Example : There is or there is not a guideline on… 8| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Level of scrutiny - Granulometry Ad Qu Sa Ef Sp A Increased number of indicators on the same subject Example : In this guideline, there is or not the following aspects: Administrative part, Quality part, Safety part, Efficacy part, Product Information 9| Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Level of scrutiny - Granulometry Qd Qv Qs Qi Comprehensive number of indicators Example : In this quality part of this guideline, there is or not the following aspects: Impurities, Stability testing for drug substance and drug product, Validation of analytical method, Pharmaceutical development, Specifications for drug substance and drug product, … 10 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

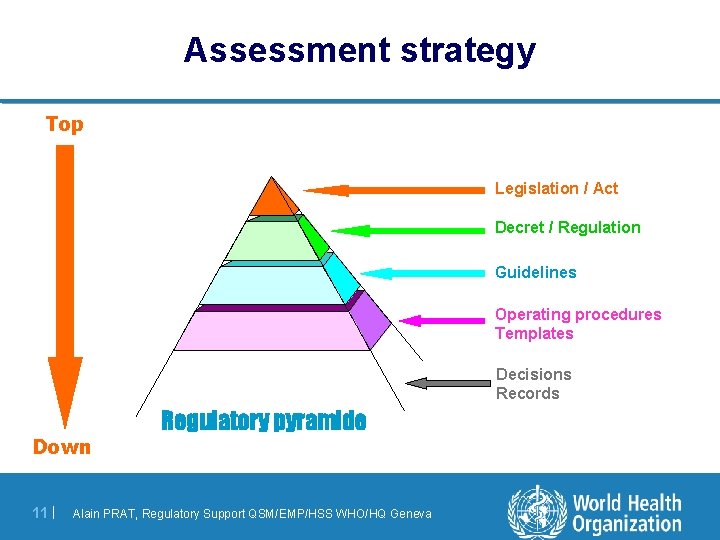
Assessment strategy Top Legislation / Act Decret / Regulation Guidelines Operating procedures Templates Decisions Records Down 11 | Regulatory pyramide Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Assessment methodology l Not based on impressions, feelings or any subjective considerations l Based on objective evidence of the existence, the implementation and the results l Evidence collected through interviews should, whenever possible, be confirmed by more objective means l Possible deficiencies or gaps should be thoroughly investigated and validated l Consensus should be reached at the end with auditees 12 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Assessment of the institution(s) l Legal basis l Governance structure l Organization in place l Quality management system l Funding l Management of human resources 13 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Assessment of the institution(s) l Independence and impartiality l Transparency and confidentiality l Management of committees and external expertise l Infrastructure and equipment l Monitoring and accountability l IT Management 14 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

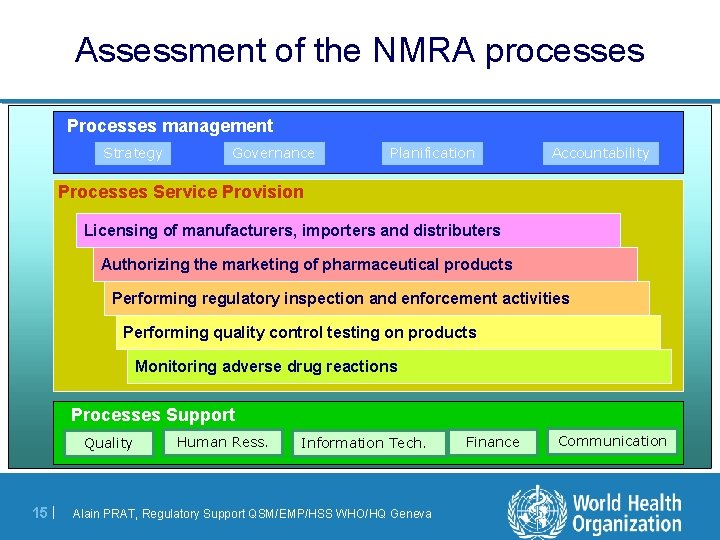
Assessment of the NMRA processes Processes management Strategy Governance Planification Accountability P ro cesses S ervi ce P ro vi si o n Licensing of manufacturers, importers and distributers Authorizing the marketing of pharmaceutical products Performing regulatory inspection and enforcement activities Performing quality control testing on products Monitoring adverse drug reactions Processes Support Quality 15 | Human Ress. Information Tech. Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva Finance Communication

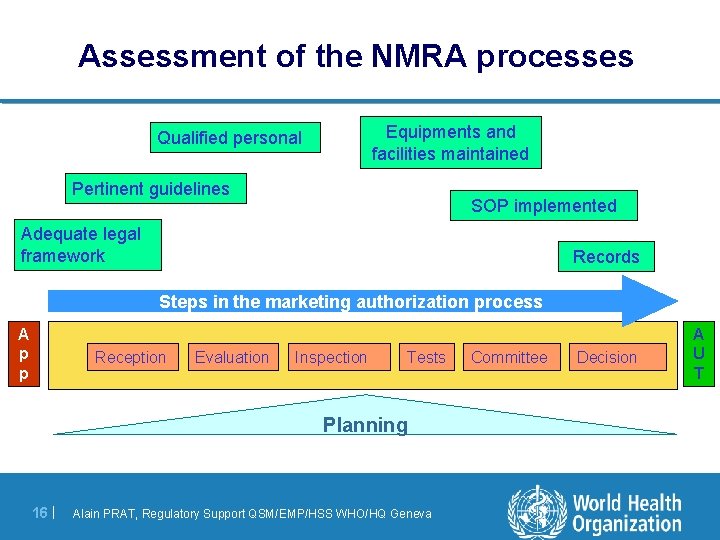
Assessment of the NMRA processes Equipments and facilities maintained Qualified personal Pertinent guidelines SOP implemented Adequate legal framework Records Steps in the marketing authorization process A p p Reception Evaluation Inspection Tests Planning 16 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva Committee Decision A U T

Provision of an assessment report l Describe the existing situation l Identify the gaps l Provision of recommendations such as : – To change laws or decrees – To develop guidelines – To reorganise and reshape the structure (centralized/decentralized activities) – To implement QMS, to develop procedures and records – To manage and planning for Human resources – To implement new approach or strategy 17 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

NMRA assessments worldwide l 54 Assessments performed on 49 Regulatory systems (with the involvement of who Headquaters) – – – AFRO - 26 COUNTRIES / 30 ASSESSMENTS EURO - 3 COUNTRIES / 3 ASSESSMENTS EMRO - 6 COUNTRIES / 7 ASSESSMENTS SEARO - 5 COUNTRIES / 5 ASSESSMENTS WPRO - 7 COUNTRIES / 7 ASSESSMENTS PAHO - 2 COUNTRIES / 2 ASSESSMENTS l WHO Regional assessments (without involving WHO Headquaters) – ? ? l Self-assessments – ? ? ? 18 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

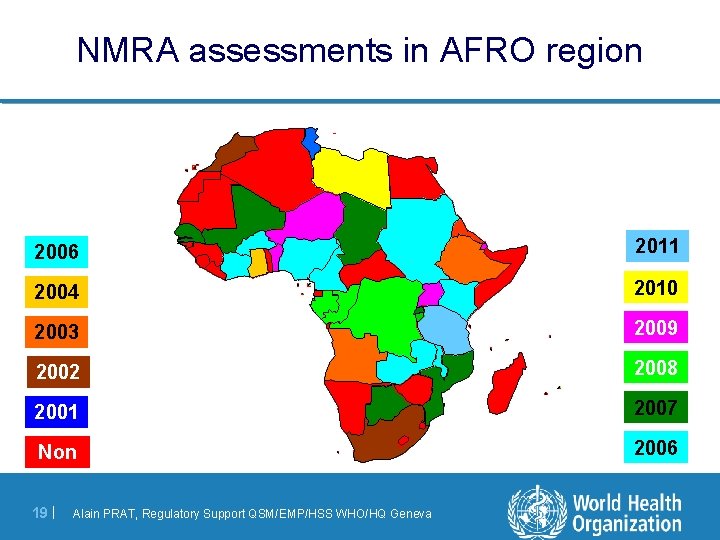
NMRA assessments in AFRO region 2006 2011 2004 2010 2003 2009 2002 2008 2001 2007 Non 2006 19 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

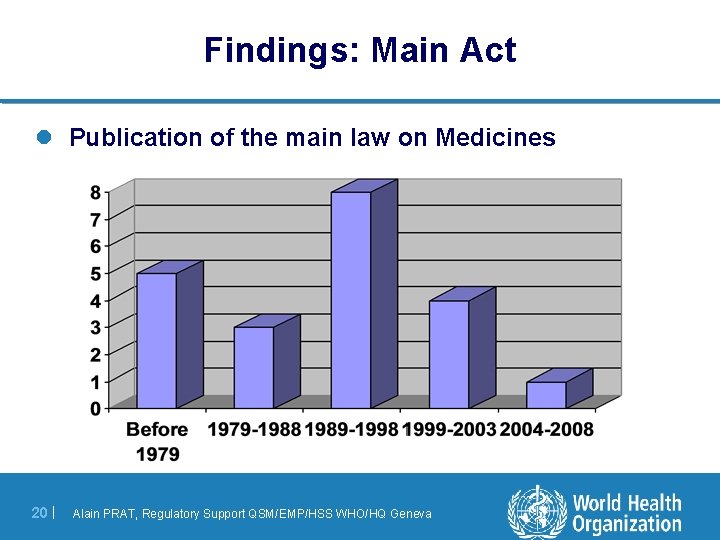
Findings: Main Act l Publication of the main law on Medicines 20 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

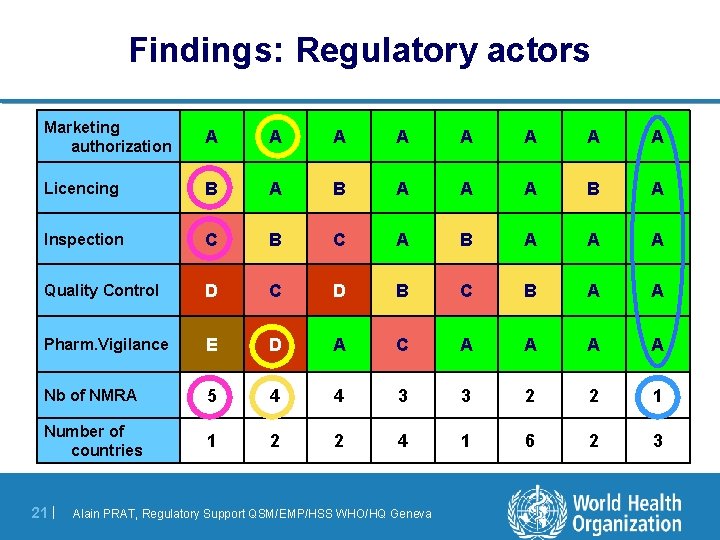
Findings: Regulatory actors Marketing authorization A A A A Licencing B A A A B A Inspection C B C A B A A A Quality Control D C D B C B A A Pharm. Vigilance E D A C A A Nb of NMRA 5 4 4 3 3 2 2 1 Number of countries 1 2 2 4 1 6 2 3 21 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

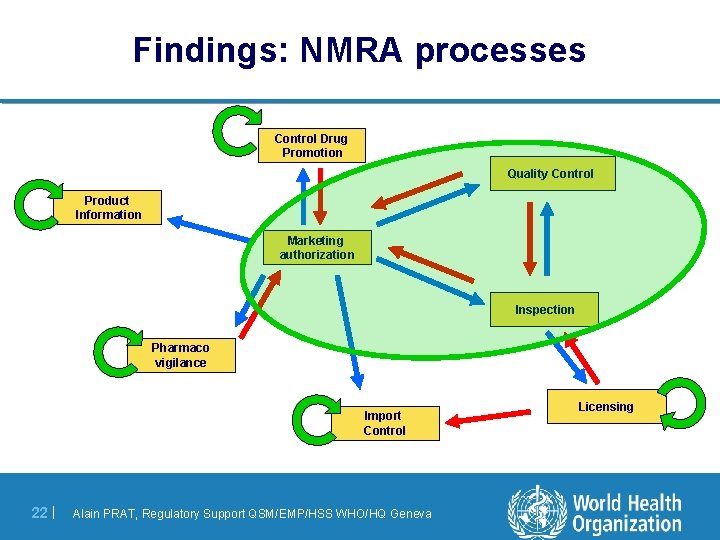
Findings: NMRA processes Control Drug Promotion Quality Control Product Information Marketing authorization Inspection Pharmaco vigilance Import Control 22 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva Licensing

Findings : Advisory committees l Committees involved in the marketing authorization processes in the AFRO region 23 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

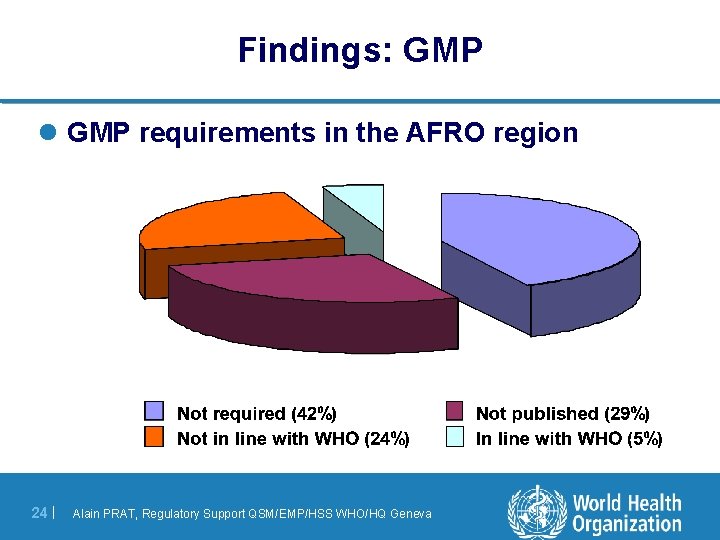
Findings: GMP l GMP requirements in the AFRO region 24 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Future improvements l Improve the tool itself – – Improve the technical content / scrutiny Improve the usability mainly using IT system Improve the assessment of the performance of the processes Build comparative features • to enable comparison of assessments outcomes conducted during a period of time on the same NMRA – Build regional features • to enable comparison of assessments outcomes conducted on several NMRAs within the same REC 25 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Future improvements l Improve the assessment process – Implement Quality management system to cover the Assessment process – Implement certification of Assessors within and without WHO to ensure the same qualification / competence of all assessors l Update and develop the references we are using – Revised WHO Guidelines on medicines regulatory systems 26 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

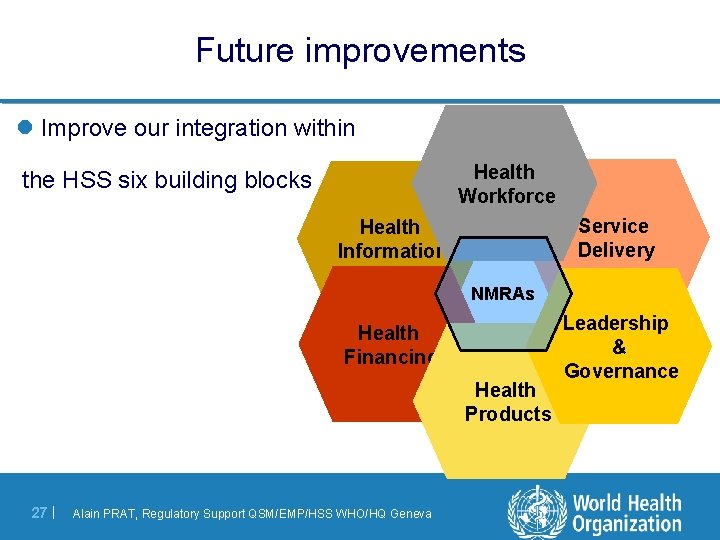
Future improvements l Improve our integration within Health Workforce the HSS six building blocks Service Delivery Health Information NMRAs Health Financing Health Products 27 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva Leadership & Governance

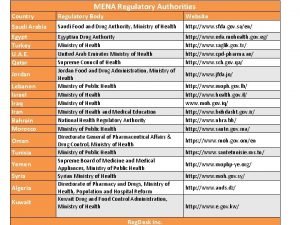
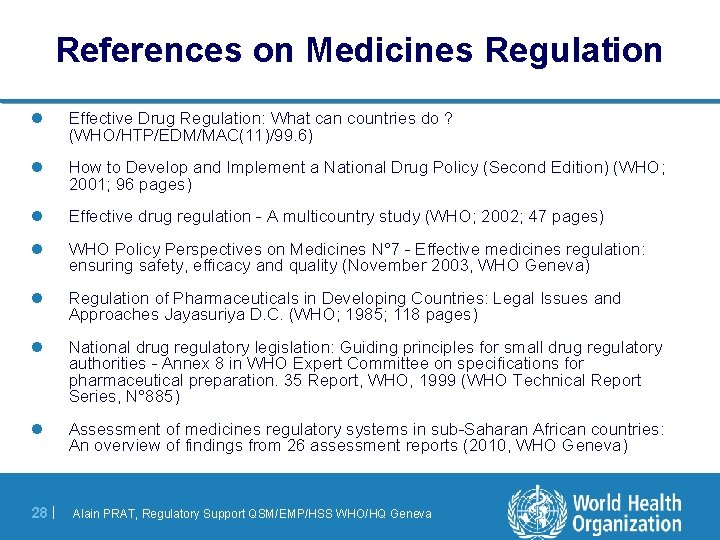
References on Medicines Regulation l Effective Drug Regulation: What can countries do ? (WHO/HTP/EDM/MAC(11)/99. 6) l How to Develop and Implement a National Drug Policy (Second Edition) (WHO; 2001; 96 pages) l Effective drug regulation - A multicountry study (WHO; 2002; 47 pages) l WHO Policy Perspectives on Medicines N° 7 - Effective medicines regulation: ensuring safety, efficacy and quality (November 2003, WHO Geneva) l Regulation of Pharmaceuticals in Developing Countries: Legal Issues and Approaches Jayasuriya D. C. (WHO; 1985; 118 pages) l National drug regulatory legislation: Guiding principles for small drug regulatory authorities - Annex 8 in WHO Expert Committee on specifications for pharmaceutical preparation. 35 Report, WHO, 1999 (WHO Technical Report Series, N° 885) l Assessment of medicines regulatory systems in sub-Saharan African countries: An overview of findings from 26 assessment reports (2010, WHO Geneva) 28 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva

Thanks for your attention 29 | Alain PRAT, Regulatory Support QSM/EMP/HSS WHO/HQ Geneva
 National medicines policy
National medicines policy Regulatory impact assessment india
Regulatory impact assessment india Veterinary medicines directorate
Veterinary medicines directorate Chapter 19 lesson 2 using medicines safely
Chapter 19 lesson 2 using medicines safely Staff of marvelous medicines
Staff of marvelous medicines Medicines management programme
Medicines management programme Complete floor stock
Complete floor stock Akapulko
Akapulko European directorate for the quality of medicines
European directorate for the quality of medicines Summarize roosevelt's approach to environmental problems
Summarize roosevelt's approach to environmental problems Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Stockley’s drug interaction
Stockley’s drug interaction Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Ratiograstin
Ratiograstin Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre Medicinescomplete martindale
Medicinescomplete martindale Pharmac special foods
Pharmac special foods Ggc medicines
Ggc medicines Federal agency for medicines and health products
Federal agency for medicines and health products Prc br 435
Prc br 435 Ahj authority having jurisdiction
Ahj authority having jurisdiction Apostles lds seniority
Apostles lds seniority European metropolitan authorities
European metropolitan authorities Asia pacific privacy authorities
Asia pacific privacy authorities Rajasthan legal service authority
Rajasthan legal service authority Authority refers to
Authority refers to Authority refers to
Authority refers to Hubs and authorities
Hubs and authorities