WHO API prequalification procedure Antony Fake Ph D







- Slides: 21

WHO API prequalification procedure Antony Fake Ph. D WHO Prequalification of Medicines Programme 1| WHO Prequalification of APIs – TBS, Oct 2012

Overview • Introduction • Prequalification Process • Achievements • Challenges 2| WHO Prequalification of APIs – TBS, Oct 2012

Abbreviations l API – Active Pharmaceutical Ingredient l FPP – Finished Pharmaceutical Product l API PQ – API Prequalification l APIMF – Active Pharmaceutical Ingredient Master File (DMF) l CPQ – Confirmation of API PQ Document l SRA – Stringent Regulatory Authority l GMP – Good Manufacturing Practice l PQP – Prequalification of Medicines Programme 3| WHO Prequalification of APIs – TBS, Oct 2012

Prequalification of Medicines website http: //www. who. int/prequal 4| WHO Prequalification of APIs – TBS, Oct 2012

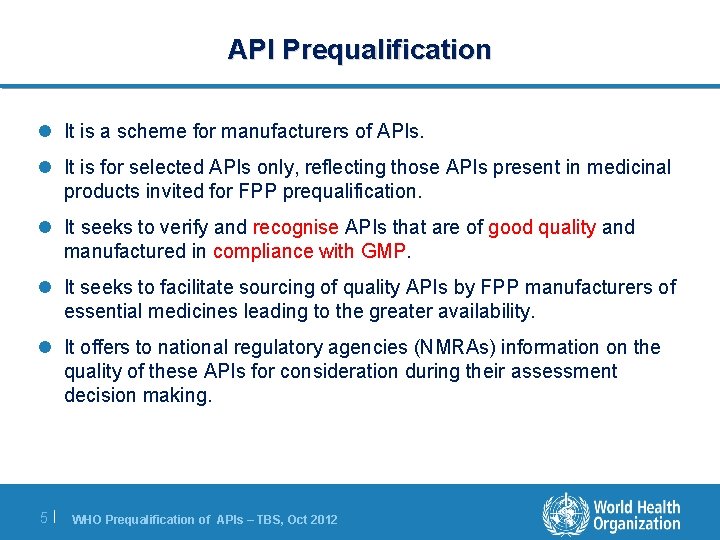
API Prequalification l It is a scheme for manufacturers of APIs. l It is for selected APIs only, reflecting those APIs present in medicinal products invited for FPP prequalification. l It seeks to verify and recognise APIs that are of good quality and manufactured in compliance with GMP. l It seeks to facilitate sourcing of quality APIs by FPP manufacturers of essential medicines leading to the greater availability. l It offers to national regulatory agencies (NMRAs) information on the quality of these APIs for consideration during their assessment decision making. 5| WHO Prequalification of APIs – TBS, Oct 2012

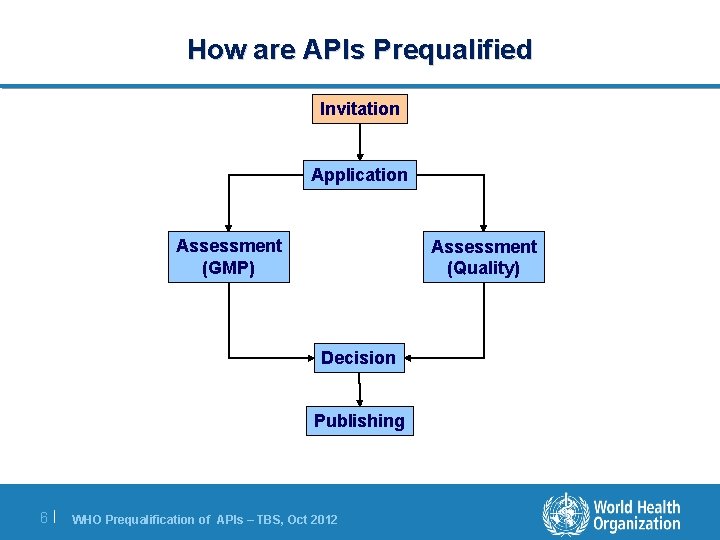
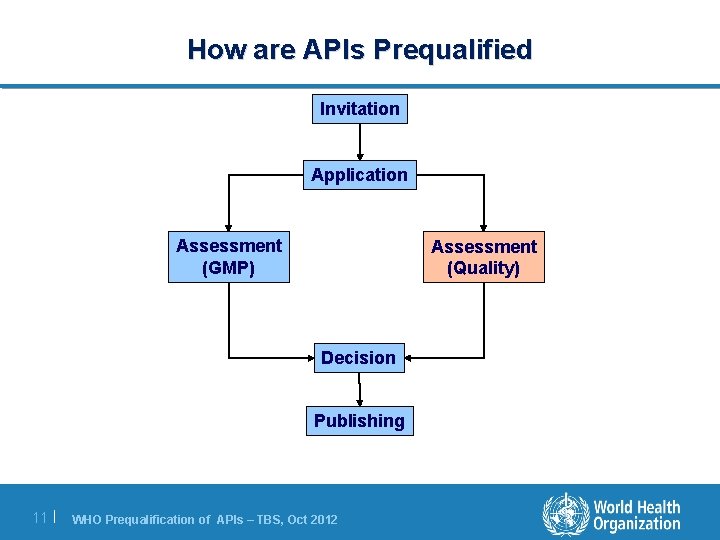
How are APIs Prequalified Invitation Application Assessment (GMP) Assessment (Quality) Decision Publishing 6| WHO Prequalification of APIs – TBS, Oct 2012

4 th Invitation for EOI HIV Anti-malarial Anti-tuberculosis Reproductive health Abacavir, Atazanavir, Darunavir, Didanosine, Efavirenz, Emtricitabine, Etravirine, Lamivudine, Lopinavir, Nevirapine, Raltegravir, Ritonavir, Stavudine, Tenofovir, Zidovudine Amodiaquine, Artemether, Artesunate, Dihydroartemisinin, Lumefantrine, Mefloquine, Piperaquine, Pyrimethamine, Sulfadoxine, Pyronaridine Amikacin, Capreomycin, Cycloserine, Ethambutol, Ethionamide, Isoniazid, Kanamycin, Levofloxacin, Moxifloxacin, Ofloxacin, Para-Aminosalicylic Acid (PAS), Prothionamide, Pyrazinamide, Rifampicin, Streptomycin, Terizidone Desogestrel, Estradiol, Ethinylestradiol, Etonogestrel, Levonorgestrel, Medroxyprogesterone, Mifepristone, Misoprostol, Norethisterone, Norgestrel, Oxytocin Neglected Tropical Diethylcarbamazine, Mebendazole Diseases Treatment of Diarrohea 7| Zinc sulphate WHO Prequalification of APIs – TBS, Oct 2012

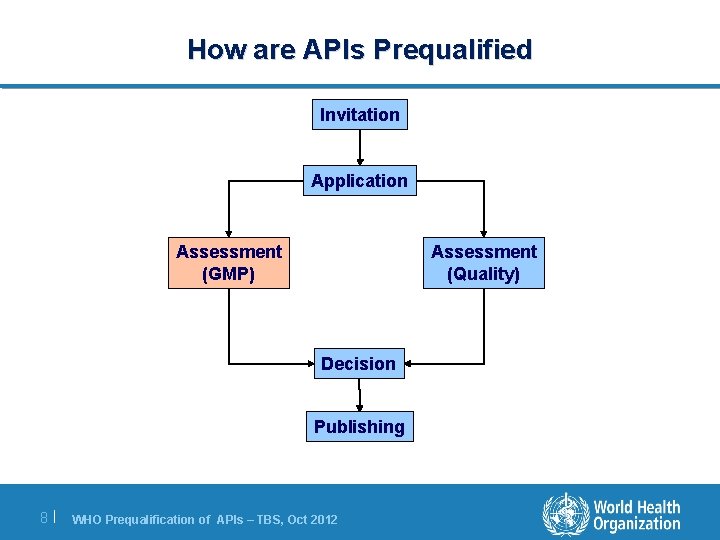
How are APIs Prequalified Invitation Application Assessment (GMP) Assessment (Quality) Decision Publishing 8| WHO Prequalification of APIs – TBS, Oct 2012

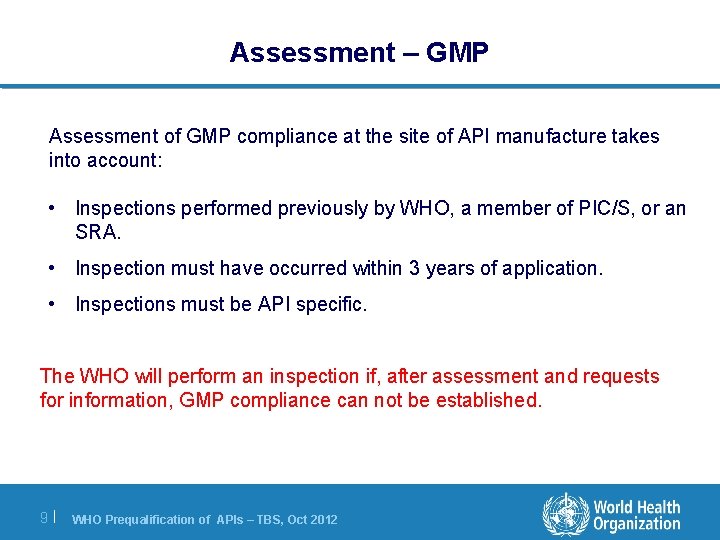
Assessment – GMP Assessment of GMP compliance at the site of API manufacture takes into account: • Inspections performed previously by WHO, a member of PIC/S, or an SRA. • Inspection must have occurred within 3 years of application. • Inspections must be API specific. The WHO will perform an inspection if, after assessment and requests for information, GMP compliance can not be established. 9| WHO Prequalification of APIs – TBS, Oct 2012

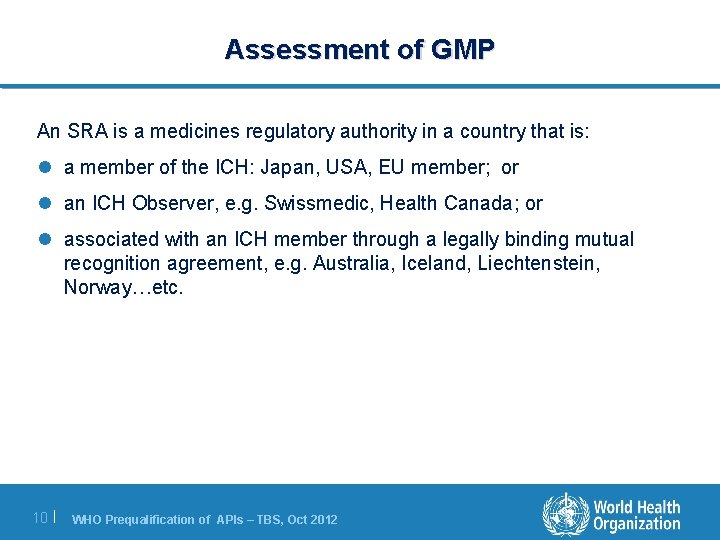
Assessment of GMP An SRA is a medicines regulatory authority in a country that is: l a member of the ICH: Japan, USA, EU member; or l an ICH Observer, e. g. Swissmedic, Health Canada; or l associated with an ICH member through a legally binding mutual recognition agreement, e. g. Australia, Iceland, Liechtenstein, Norway…etc. 10 | WHO Prequalification of APIs – TBS, Oct 2012

How are APIs Prequalified Invitation Application Assessment (GMP) Assessment (Quality) Decision Publishing 11 | WHO Prequalification of APIs – TBS, Oct 2012

Quality Assessment Involves the assessment of the APIMF (Drug Master File) submitted by the applicant. Expected technical content and assessment approach follows key international guidance and can be located in the published guideline : Guideline on submission of documentation for a multisource (generic) finished pharmaceutical product (FPP): quality part. http: //www. who. int/prequal/info_general/documents/generic_guide/Generic. G uideline_Quality. pdf. In some cases this assessment has already been completed for APIMFs already used in the FPP prequalification procedure. 12 | WHO Prequalification of APIs – TBS, Oct 2012

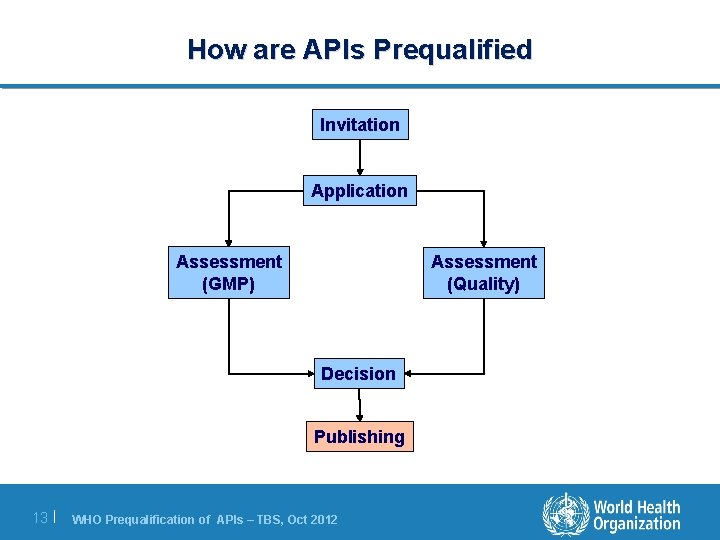
How are APIs Prequalified Invitation Application Assessment (GMP) Assessment (Quality) Decision Publishing 13 | WHO Prequalification of APIs – TBS, Oct 2012

Publishing + List of PQ APIs Website (Public) WHOPIRs Website (Public) Confirmation Document (Private) 14 | WHO Prequalification of APIs – TBS, Oct 2012

WHO List of Prequalified APIs Publically available ● ● ● ● WHO reference number. ● The primary and secondary packaging components. INN name. ● The assigned re-test period. Date of prequalification. Name of the applicant ● The recommended storage conditions. Sites of API manufacture. The APIMF version number. ● Confirmation of API PQ document issue date The API specification version number. Intended for: UN agencies, National medicine authorities, FPP manufacturers, public 15 | WHO Prequalification of APIs – TBS, Oct 2012

Confirmation of API PQ document Provided to the API manufacturer for distribution at their discretion l The assigned WHO reference number. l The INN name of the active pharmaceutical ingredient. l API manufacturer company name. l The API specification version number. l A copy of the API specifications. l The assigned re-test period. l The recommended storage conditions. l A copy of the assay and related substances test methods. Intended for: UN agencies, National medicine authorities, FPP manufacturers 16 | WHO Prequalification of APIs – TBS, Oct 2012

API Prequalification Successes 1 April 2011 1 November 2011 October 2012 Total number of applications 8 29 64 Number of PQ APIs 2 5 23 HIV Malaria TB RH Total number of 17 applications 26 16 5 Number of PQ APIs 15 6 - 17 | 2 WHO Prequalification of APIs – TBS, Oct 2012

API Prequalification Successes • Roughly half of the applications made used APIMFs not previously used in support of PQ FPPs. • Half of these new applications came from China, compared to less than 10% in support of PQ FPPs. • Submission of applications from emerging manufacturers. 18 | WHO Prequalification of APIs – TBS, Oct 2012

API Prequalification Successes • A higher than expected number of submission of applications in RH area. • It is noted that there are submissions of applications from manufacturers who currently manufacture FPPs domestically. • Recognition of API PQ as one means of satisfying API requirements in Malaysia. • Sharing of an assessment report from the EDQM to permit an abridged assessment. 19 | WHO Prequalification of APIs – TBS, Oct 2012

Challenges • Increasing recognition of the PQ scheme among manufacturers. • Maximising the value of the PQ assessments for national regulatory agencies - Increasing the value of API PQ to manufacturers. • Maximising use existing evidence of Quality and GMP for manufacturers seeking prequalification. • Formalising application processes – dealing with increased workload. 20 | WHO Prequalification of APIs – TBS, Oct 2012

Thank you If you have any further questions, or You have ideas or are interested in greater involvement in API PQ please feel free to contact me at: Fakea@who. int 21 | WHO Prequalification of APIs – TBS, Oct 2012