WHMIS workplace hazardous materials information system all chemicals


















- Slides: 44

WHMIS ¬ workplace hazardous materials information system ¬all chemicals are treated with respect ¬ WHMIS has been developed to provide guidelines for handling, storage and disposal of reactive materials ¬ MSDS is a material safety data sheet

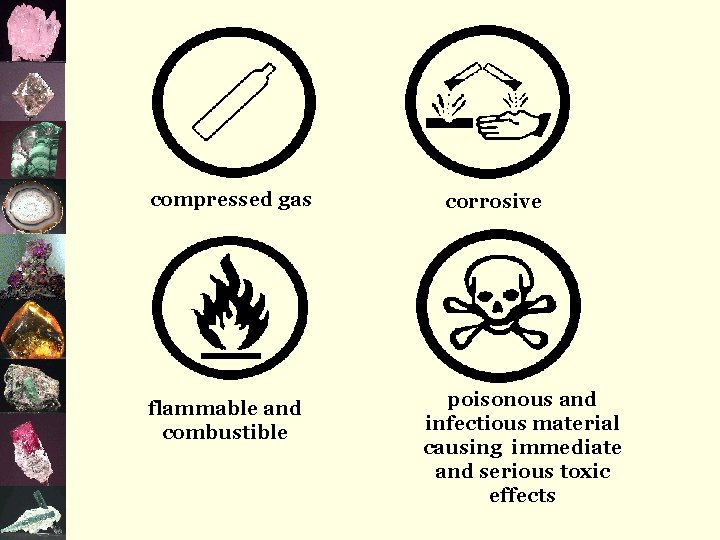
compressed gas flammable and combustible corrosive poisonous and infectious material causing immediate and serious toxic effects

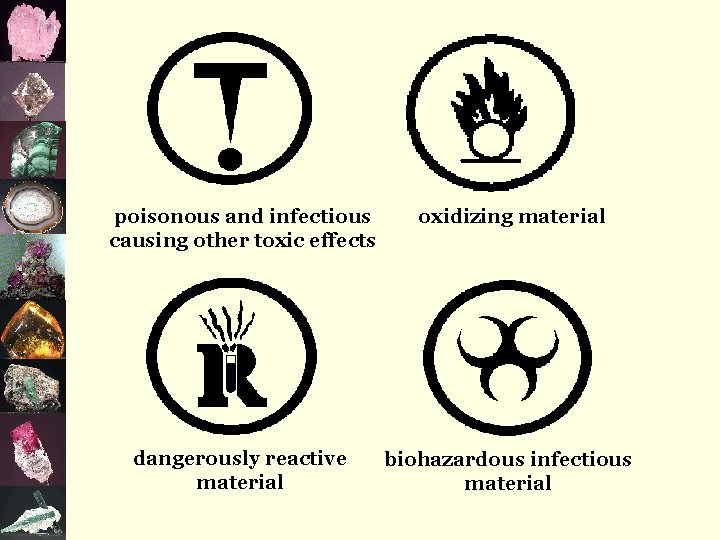
poisonous and infectious causing other toxic effects oxidizing material dangerously reactive material biohazardous infectious material

Science 10 Review A. The Atom ¬ proton p+ positive charge ¬ neutron n 0 zero charge ¬ electron e– negative charge ¬ electrons are found in a cloud region around the nucleus ¬ nucleus contains the protons and neutrons which make up most of mass of atom

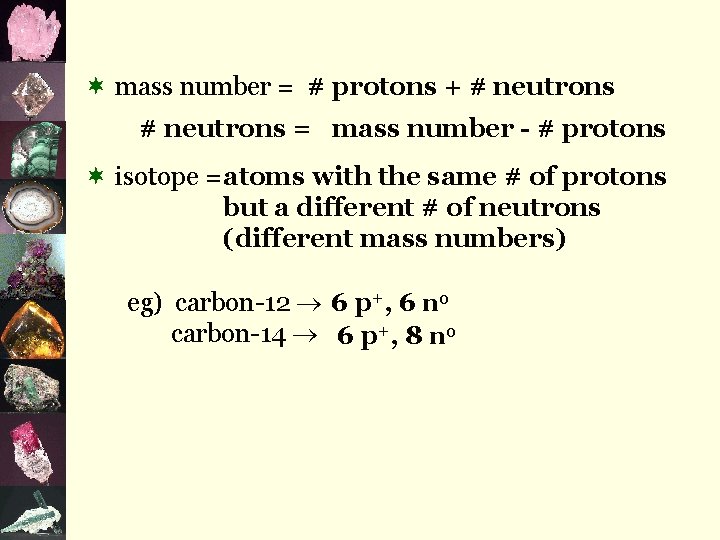
¬ mass number = # protons + # neutrons = mass number - # protons ¬ isotope = atoms with the same # of protons but a different # of neutrons (different mass numbers) eg) carbon-12 6 p+, 6 n 0 carbon-14 6 p+, 8 n 0

B. Periodic Table ¬arranged in groups (columns) and periods (rows) ¬group number = number of outer level (valence) e¬period number = number of energy levels occupied by e. Ex. Na Group number = 1 Period number = 3 This info is helpful for drawing Energy Level Diagrams

C. Energy Level Diagrams ¬atoms are electrically neutral that the # of p+ = # of e- which means ¬maximum number of e-: 3 rd level = 8 e 2 nd level = 8 e 1 st level = 2 e-

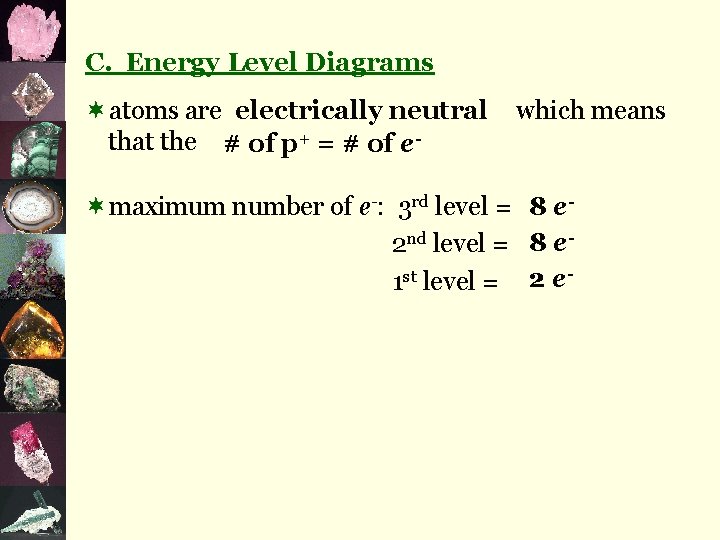
Examples 1 e 8 e 2 e- 11 p+ 3 p+ Na Li

D. Ions ¬ions are particles or groups of particles that have a net charge (either positive or negative) ¬neutral atoms are unstable if their valence level is not full ¬atoms will strive to satisfy the octet rule in order to become stable…in other words, they strive to have a full valence level and do so by giving away or taking e-
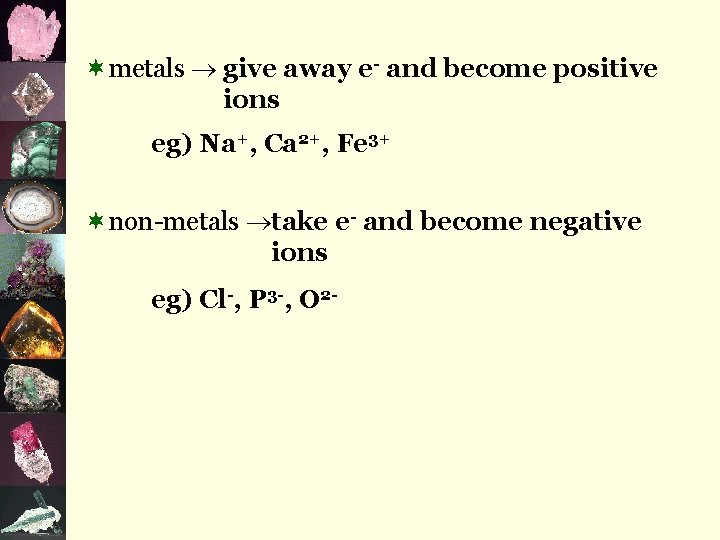
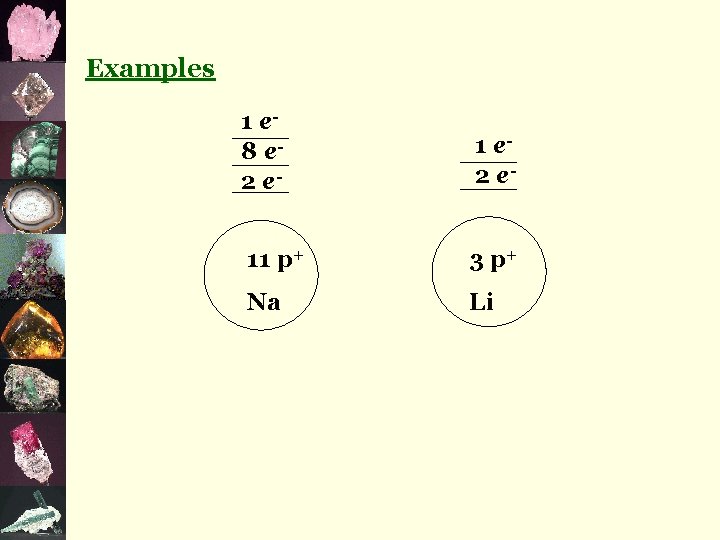
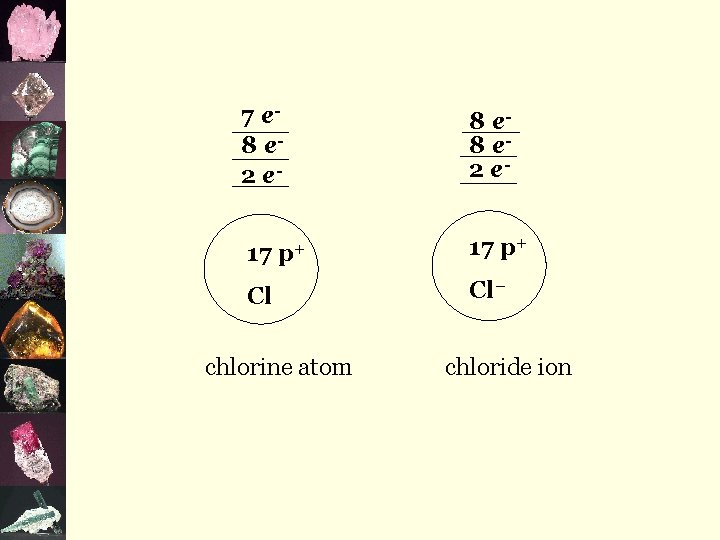
¬metals give away e- and become positive ions eg) Na+, Ca 2+, Fe 3+ ¬non-metals take e- and become negative ions eg) Cl-, P 3 -, O 2 -

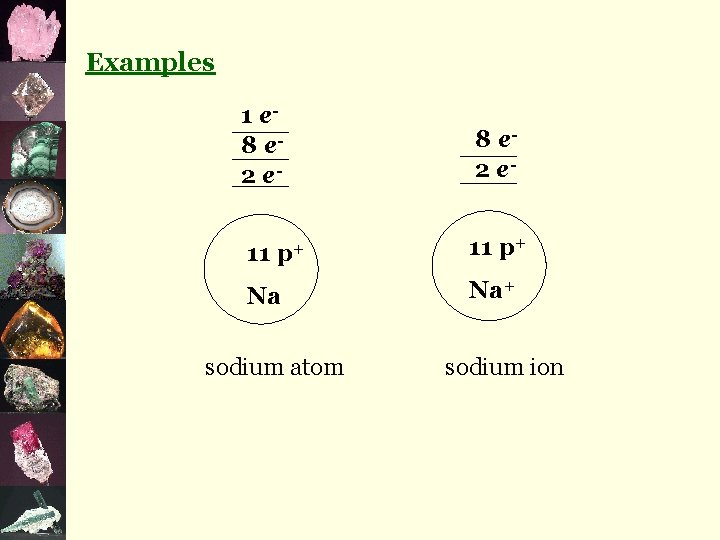
Examples 1 e 8 e 2 e 11 p+ Na sodium atom 8 e 2 e 11 p+ Na+ sodium ion

7 e 8 e 2 e 17 p+ Cl chlorine atom 8 e 2 e 17 p+ Cl– chloride ion

Your Assignment: pgs 1 -3 in workbook

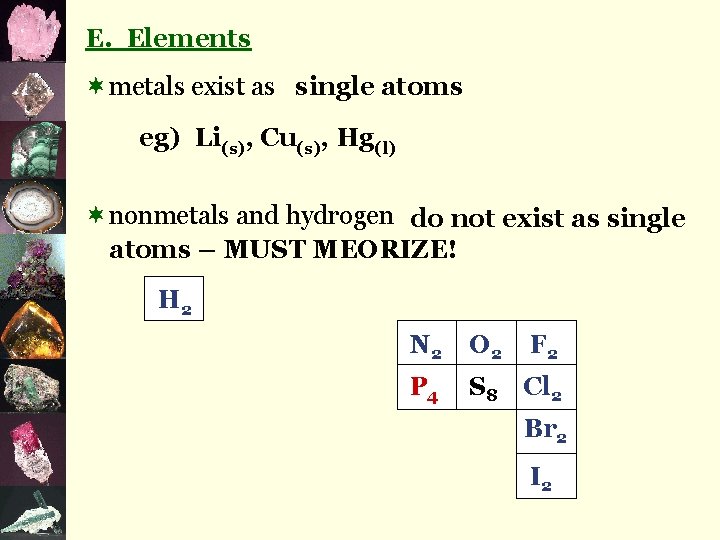
E. Elements ¬metals exist as single atoms eg) Li(s), Cu(s), Hg(l) ¬nonmetals and hydrogen do not exist as single atoms – MUST MEORIZE! H 2 N 2 O 2 F 2 P 4 S 8 Cl 2 Br 2 I 2

Try These: 1. Cu(s) = copper 2. O 2(g) = oxygen gas 3. Al(s) = aluminum 4. fluorine gas = F 2(g) 5. barium = Ba(s) 6. nitrogen gas = N 2(g)

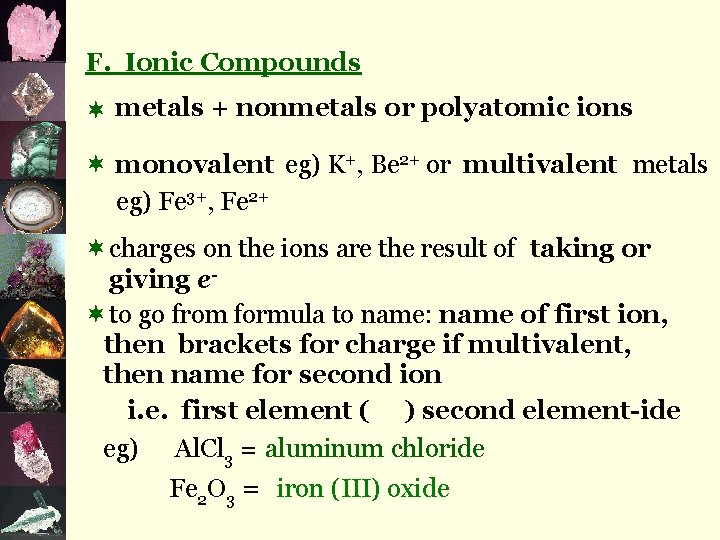
F. Ionic Compounds ¬ metals + nonmetals or polyatomic ions ¬ monovalent eg) K+, Be 2+ or multivalent metals eg) Fe 3+, Fe 2+ ¬charges on the ions are the result of taking or giving e¬to go from formula to name: name of first ion, then brackets for charge if multivalent, then name for second ion i. e. first element ( ) second element-ide eg) Al. Cl 3 = aluminum chloride Fe 2 O 3 = iron (III) oxide

Try These: 1. Zn 3 P 2 = zinc phosphide 2. Na. NO 3 = sodium nitrate 3. Ni. F 3 = nickel (III) fluoride 4. Mn. O 2 = manganese (IV) oxide 5. Cr 2(SO 4)3 = chromium (III) sulphate

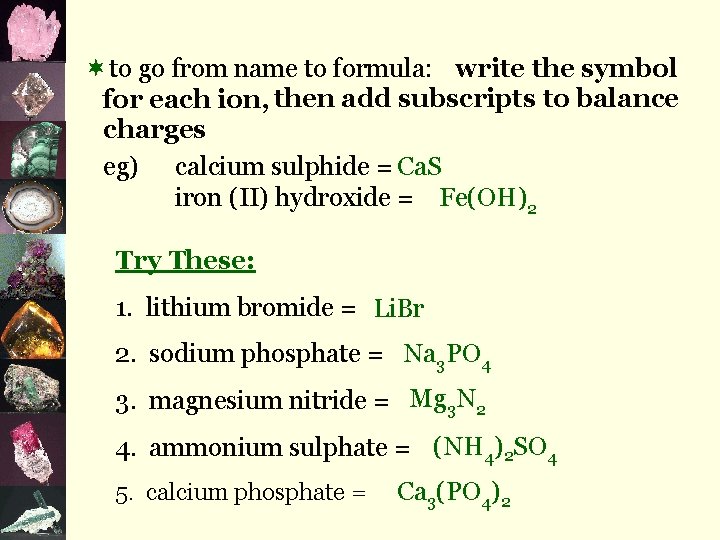
¬to go from name to formula: write the symbol for each ion, then add subscripts to balance charges eg) calcium sulphide = Ca. S iron (II) hydroxide = Fe(OH)2 Try These: 1. lithium bromide = Li. Br 2. sodium phosphate = Na 3 PO 4 3. magnesium nitride = Mg 3 N 2 4. ammonium sulphate = (NH 4)2 SO 4 5. calcium phosphate = Ca 3(PO 4)2

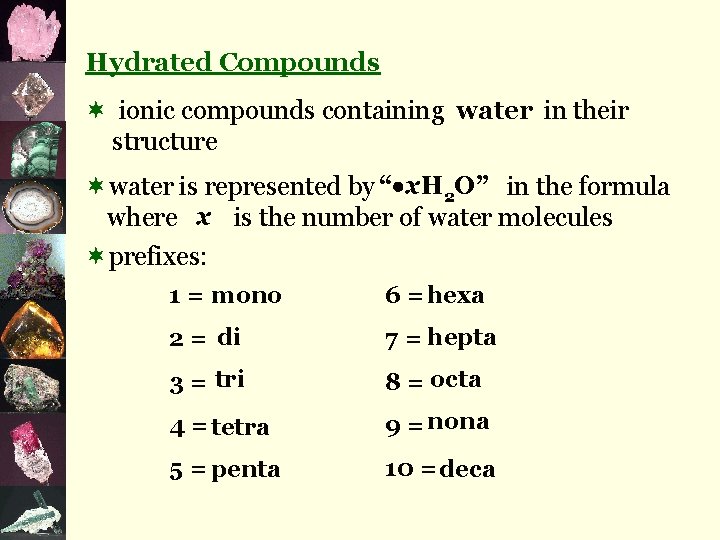
Hydrated Compounds ¬ ionic compounds containing water in their structure ¬water is represented by “ x. H 2 O” in the formula where x is the number of water molecules ¬prefixes: 1 = mono 6 = hexa 2 = di 7 = hepta 3 = tri 8 = octa 4 = tetra 9 = nona 5 = penta 10 = deca

¬to go from name to formula: give the ionic name for the first part of the compound, then name the “ x. H 2 O” part as prefix + “hydrate” eg) Na. F 3 H 2 O = sodium fluoride trihydrate Cu. SO 4 5 H 2 O = copper (II) sulphate pentahydrate

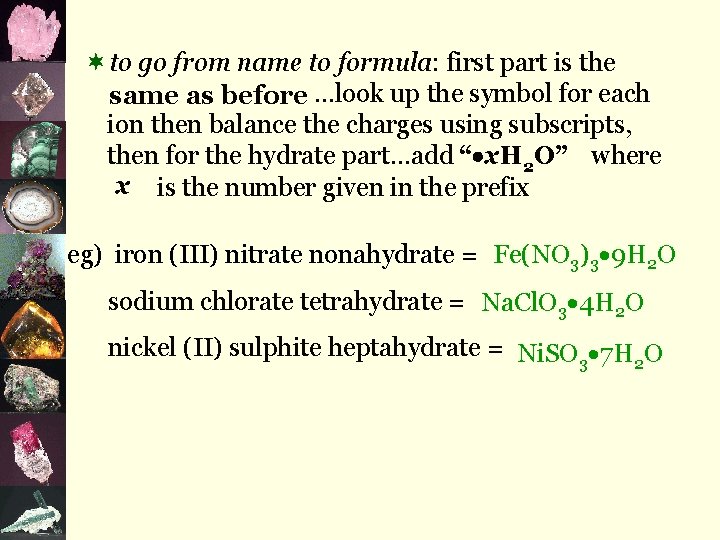
¬to go from name to formula: first part is the same as before …look up the symbol for each ion then balance the charges using subscripts, then for the hydrate part…add “ x. H 2 O” where x is the number given in the prefix eg) iron (III) nitrate nonahydrate = Fe(NO 3)3 9 H 2 O sodium chlorate tetrahydrate = Na. Cl. O 3 4 H 2 O nickel (II) sulphite heptahydrate = Ni. SO 3 7 H 2 O

Your Assignment: pgs 3, 4 in workbook

G. Molecular Compounds ¬ nonmetals only ¬ e- are shared therefore no ions are formed ¬ no charges involved ¬ use prefixes in naming

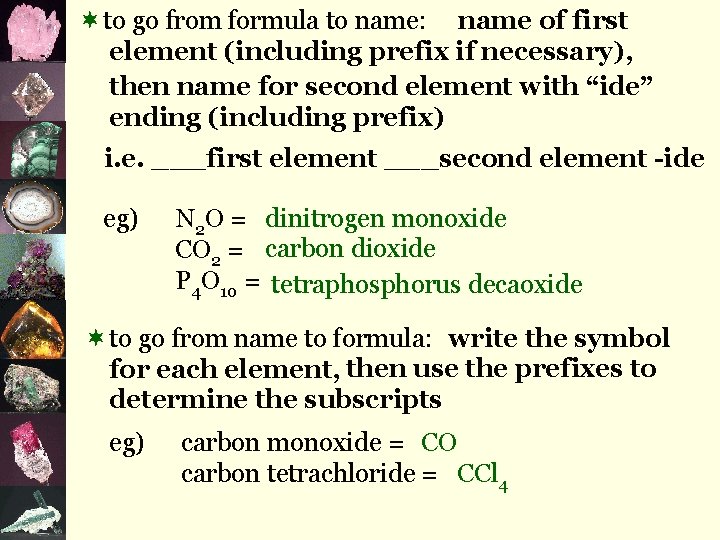
¬to go from formula to name: name of first element (including prefix if necessary), then name for second element with “ide” ending (including prefix) i. e. ___first element ___second element -ide eg) N 2 O = dinitrogen monoxide CO 2 = carbon dioxide P 4 O 10 = tetraphosphorus decaoxide ¬to go from name to formula: write the symbol for each element, then use the prefixes to determine the subscripts eg) carbon monoxide = CO carbon tetrachloride = CCl 4

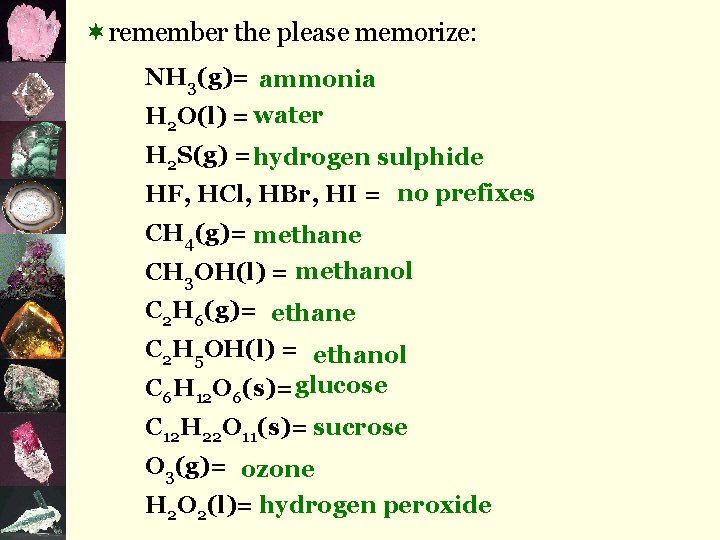
¬remember the please memorize: NH 3(g)= ammonia H 2 O(l) = water H 2 S(g) = hydrogen sulphide HF, HCl, HBr, HI = no prefixes CH 4(g)= methane CH 3 OH(l) = methanol C 2 H 6(g)= ethane C 2 H 5 OH(l) = ethanol C 6 H 12 O 6(s)= glucose C 12 H 22 O 11(s)= sucrose O 3(g)= ozone H 2 O 2(l)= hydrogen peroxide

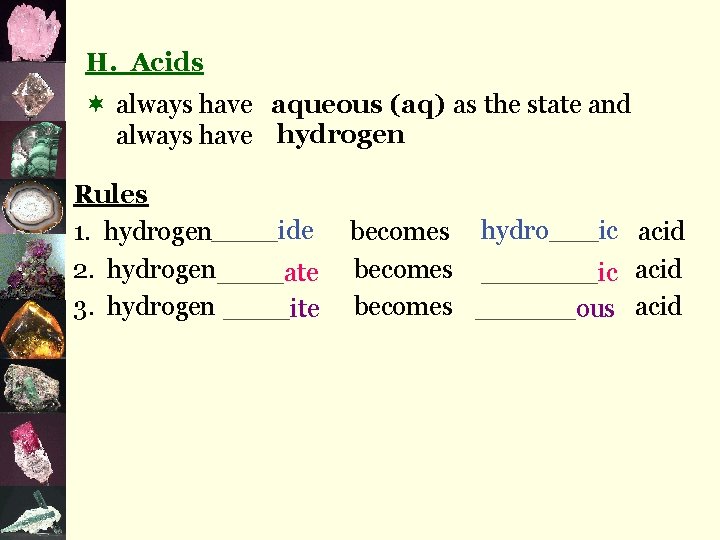
H. Acids ¬ always have aqueous (aq) as the state and always have hydrogen Rules 1. hydrogen____ide 2. hydrogen ____ate 3. hydrogen ____ite becomes hydro___ic acid becomes ______ous acid

Try These: 1. hydrogen iodide =hydroiodic acid HI(aq) 2. hydrogen phosphate = phosphoric acid H 3 PO 4(aq) 3. hydrogen nitrite = nitrous acid HNO 2(aq) 4. hydrogen sulphite = sulphurous acid H 2 SO 3(aq)

Your Assignment: pgs 5 -7 in workbook

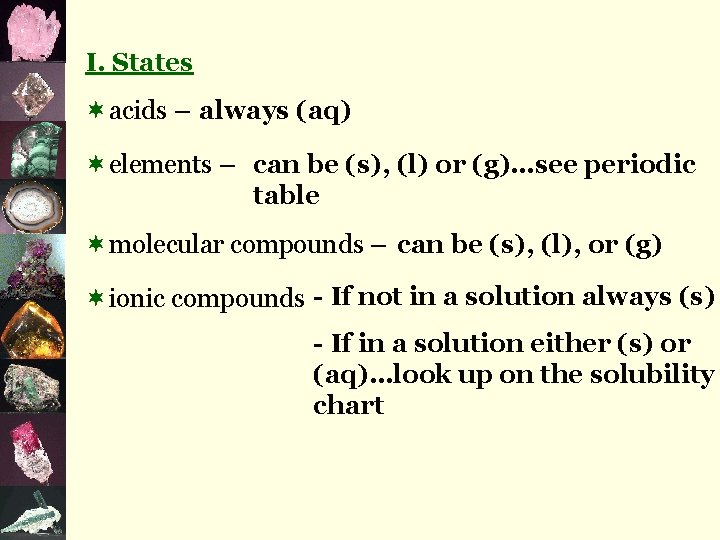
I. States ¬acids – always (aq) ¬elements – can be (s), (l) or (g)…see periodic table ¬molecular compounds – can be (s), (l), or (g) ¬ionic compounds - If not in a solution always (s) - If in a solution either (s) or (aq)…look up on the solubility chart

Try These: 1. Na. CH 3 COO(aq ) 6. Ca. CO 3( s ) 2. Ba. SO 4( s ) 7. Fe. SO 4( aq) 3. KOH( aq) 8. (NH 4)2 S( aq ) 4. Pb(NO 3)4( aq ) 9. Pb(SO 4)2( aq ) 5. Hg(CH 3 COO)2(aq ) 10. Ca 3(PO 4)2( s )

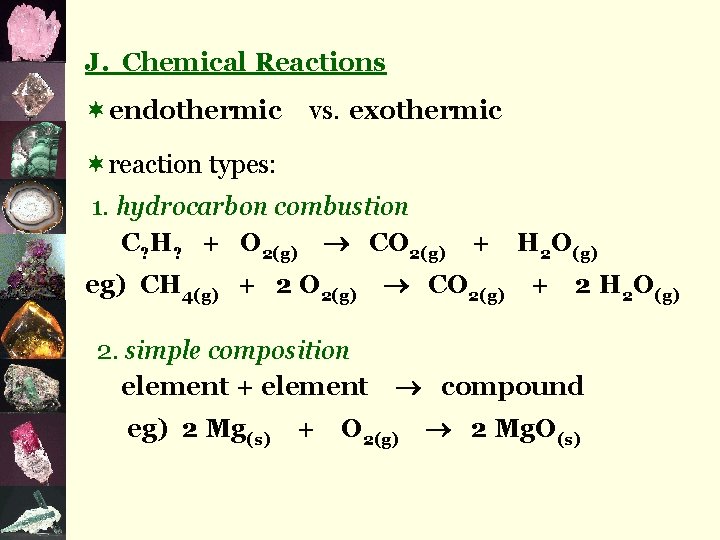
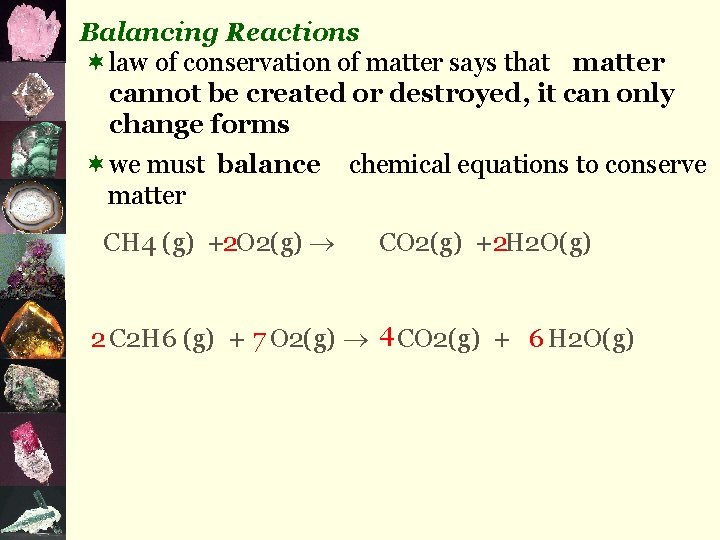
J. Chemical Reactions ¬endothermic vs. exothermic ¬reaction types: 1. hydrocarbon combustion C? H? + O 2(g) CO 2(g) eg) CH 4(g) + 2 O 2(g) 2. simple composition element + element eg) 2 Mg(s) + + CO 2(g) H 2 O(g) + 2 H 2 O(g) compound O 2(g) 2 Mg. O(s)

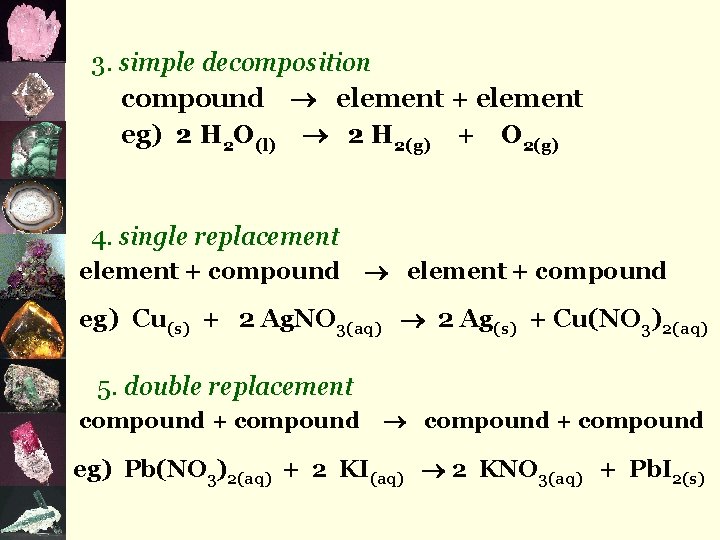
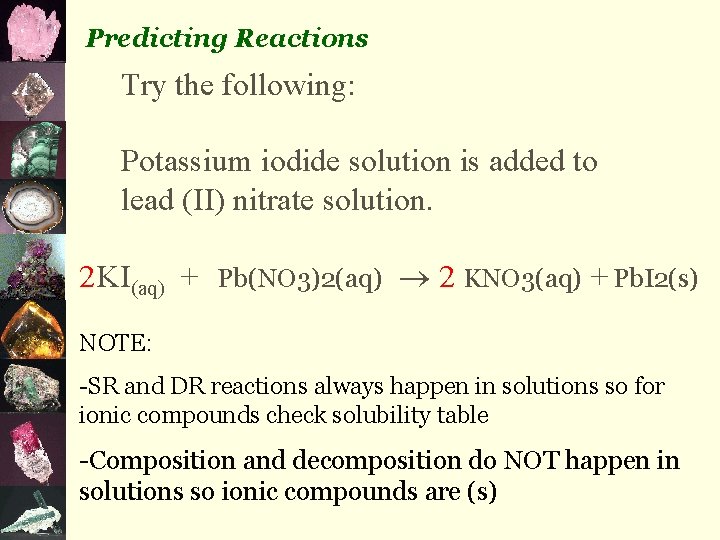
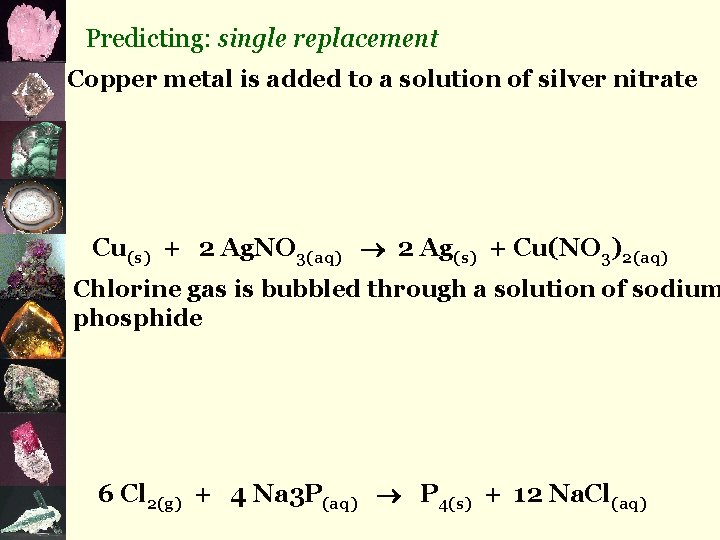
3. simple decomposition compound element + element eg) 2 H 2 O(l) 2 H 2(g) + O 2(g) 4. single replacement element + compound eg) Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) 5. double replacement compound + compound eg) Pb(NO 3)2(aq) + 2 KI(aq) 2 KNO 3(aq) + Pb. I 2(s)

Balancing Reactions ¬law of conservation of matter says that matter cannot be created or destroyed, it can only change forms ¬we must balance matter CH 4 (g) +2 O 2(g) chemical equations to conserve CO 2(g) + 2 H 2 O(g) 2 C 2 H 6 (g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(g)

Your Assignment: pg 8, 1 st half p. 9

Predicting Reactions Try the following: Potassium iodide solution is added to lead (II) nitrate solution. 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) NOTE: -SR and DR reactions always happen in solutions so for ionic compounds check solubility table -Composition and decomposition do NOT happen in solutions so ionic compounds are (s)

Predicting: single replacement Copper metal is added to a solution of silver nitrate Cu(s) + 2 Ag. NO 3(aq) 2 Ag(s) + Cu(NO 3)2(aq) Chlorine gas is bubbled through a solution of sodium phosphide 6 Cl 2(g) + 4 Na 3 P(aq) P 4(s) + 12 Na. Cl(aq)

Your Assignment: pg 2 nd half p. 9

K. Significant Digits ¬any digit from 1 -9 is significant ¬trailing zeros are significant eg) 6. 3800, 12 000 ¬“sandwich” zeros are significant eg) 2. 04, 1005. 002 ¬leading zeros are not significant eg) 0. 0065 ¬ counted objects and constants are not included in sig digs

¬ / : multiply or divide then round answer to the lowest number of sig digs ¬+/ : add or subtract then round answer to the lowest number of decimal places

L. The Mole ¬it is a number= 6. 02 x 1023 “items” 1. Molar Mass ¬sum of the individual atomic masses for each element in a compound eg) CO 2 = 44. 01 g/mol Al(OH)3 = 78. 01 g/mol Cu(Cl. O 3)2 = 230. 45 g/mol

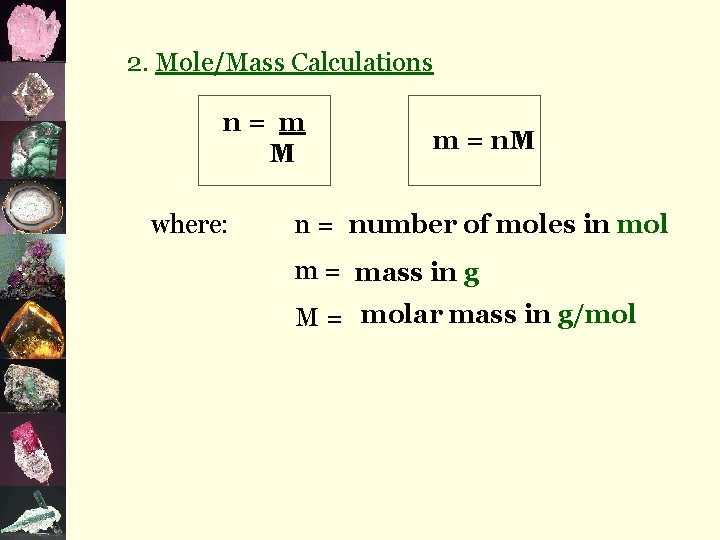
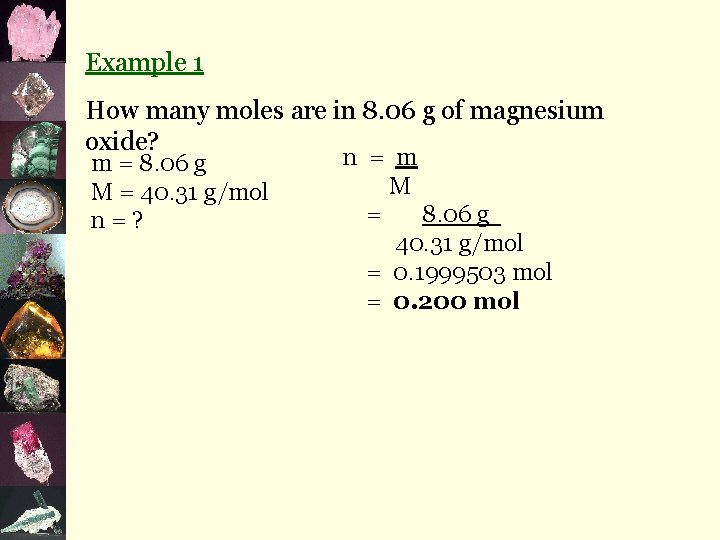
2. Mole/Mass Calculations n= m M where: m = n. M n = number of moles in mol m = mass in g M = molar mass in g/mol

Example 1 How many moles are in 8. 06 g of magnesium oxide? m = 8. 06 g M = 40. 31 g/mol n=? n = m M = 8. 06 g 40. 31 g/mol = 0. 1999503 mol = 0. 200 mol

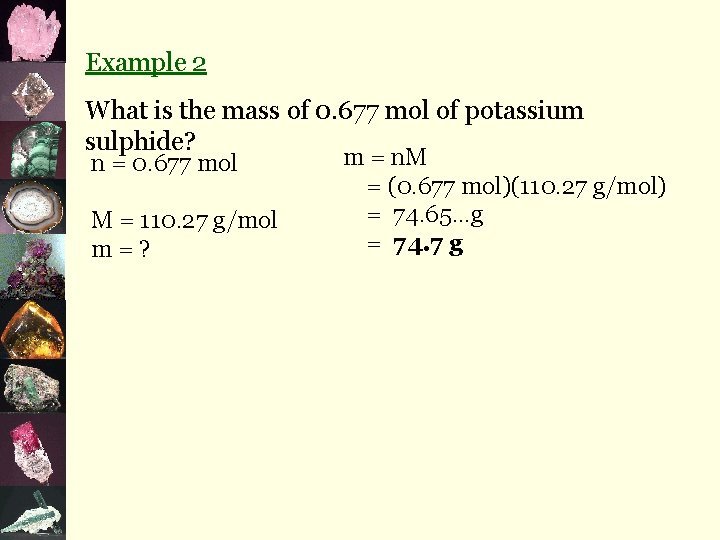
Example 2 What is the mass of 0. 677 mol of potassium sulphide? n = 0. 677 mol M = 110. 27 g/mol m=? m = n. M = (0. 677 mol)(110. 27 g/mol) = 74. 65…g = 74. 7 g

Your Assignment: p. 10 & 1 st half p. 11 Your Review Assignment: finish p. 11 – p. 13