WHMIS AND THE CLASSIFICATION OF MATTER Science 8















- Slides: 19

WHMIS AND THE CLASSIFICATION OF MATTER Science 8

WHAT DO YOU THINK WHMIS STANDS FOR? Workplace Hazardous Materials Information System

HOW TO BE SAFE IN A SCIENCE CLASSROOM THINGS TO BE AWARE OF BEFORE ENTERING A LAB HOW YOU SHOULD BE DRESSED DURING A LAB • Exits • NO contacts • Fire Alarm • Safety glasses • Fire Extinguisher • Closed toed shoes • Eye wash station • No “dangling” jewelry • Fire blanket • Hair tied back • Info on chemicals • No baggy clothes

HOW SHOULD YOU ACT IN THE LAB HOW SHOULD YOU WORK WITH OPEN FLAMES • No horseplay • Know how to put it out • Follow all instructions • No flammables nearby HOW YOU SHOULD WORK WITH CHEMICALS • No direct touching or smelling unless teacher says that it is okay • Wash skin with SOAP if contact occurs • Flush out eyes if contact occurs HOW YOU SHOULD CLEAN UP SPILLS • Tell teacher first while someone stays with the spill • Wipe away

WHAT SHOULD YOU DO IF YOU BREAK GLASSWARE WHAT WILL HAPPEN IF YOU ACT IRRESPONSIBLY • Tell teacher first while someone stays with it • Kicked out of lab, but report still due • Sweep glass, wipe with moist paper towel • Kicked out of all future labs • Never clean yourself, have the teacher do it!

WHMIS MATCHING

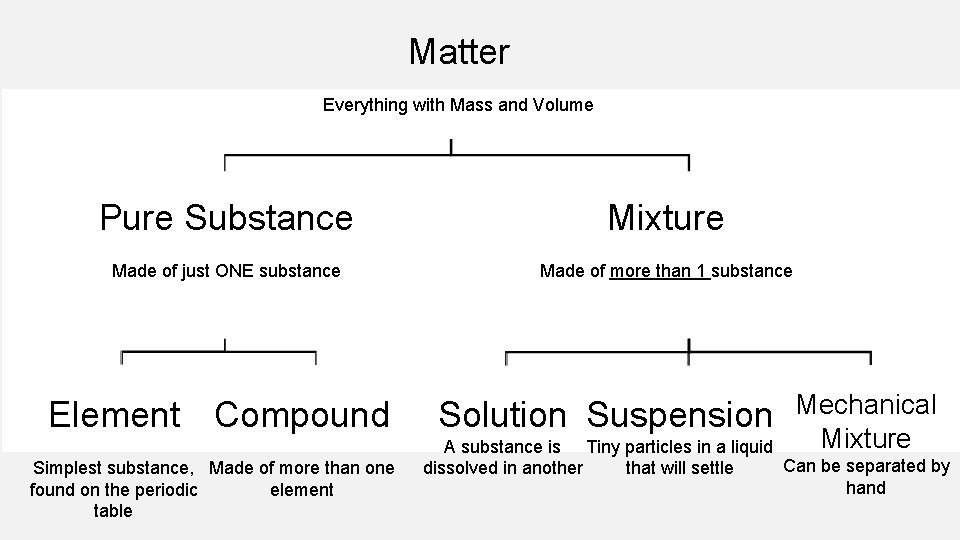
CLASSIFICATION OF MATTER

• Anything that has mass and volume is referred to as matter. • Basically anything that has a weight and size is considered matter.

Matter Everything with Mass and Volume Pure Substance Mixture Made of just ONE substance Made of more than 1 substance Element Compound Simplest substance, Made of more than one element found on the periodic table Solution Suspension Mechanical Mixture A substance is Tiny particles in a liquid Can be separated by that will settle dissolved in another hand

DESCRIBING MATTER USING PHYSICAL PROPERTIES A physical property is something you can observe without changing the matter you are observing into something else What are some physical properties we would be interested in when describing matter?

MELTING AND BOILING POINT The temperature the substance either melts or boils at

CONDUCTIVITY How well electricity moves through it

SOLUBILITY How well something dissolves

HARDNESS How resistant something is to scratches

LUSTRE Reflects light, shiny

VISCOSITY How thick a liquid is, how much it resists flow

MALLEABILITY Capable of being beat into a flat sheet

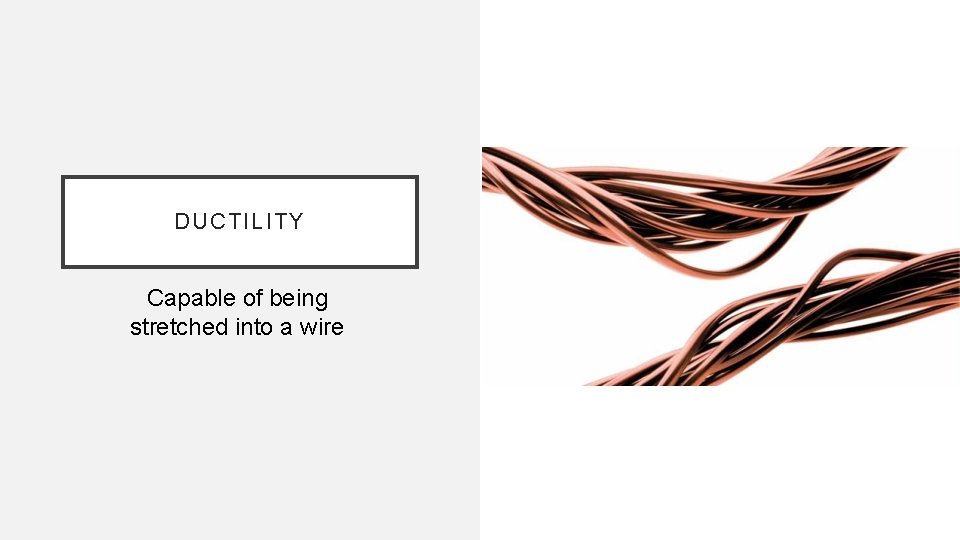
DUCTILITY Capable of being stretched into a wire

DENSITY How tightly packed something is Density = Mass / Volume