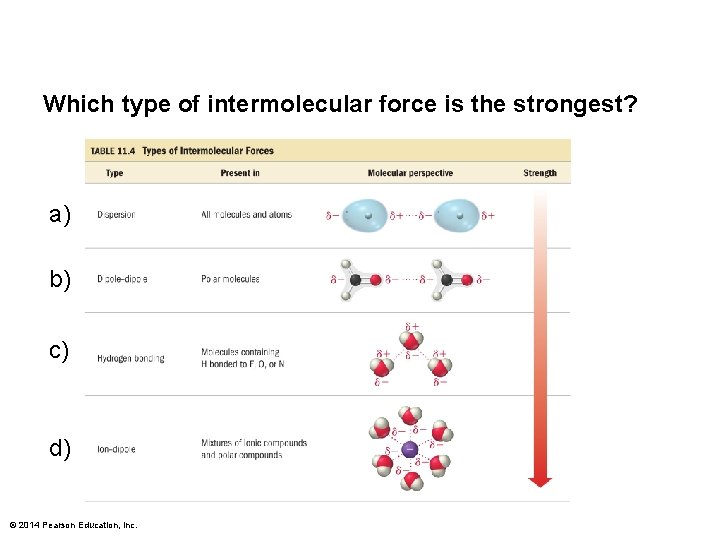
Which type of intermolecular force is the strongest
- Slides: 22

Which type of intermolecular force is the strongest? a) b) c) d) © 2014 Pearson Education, Inc.

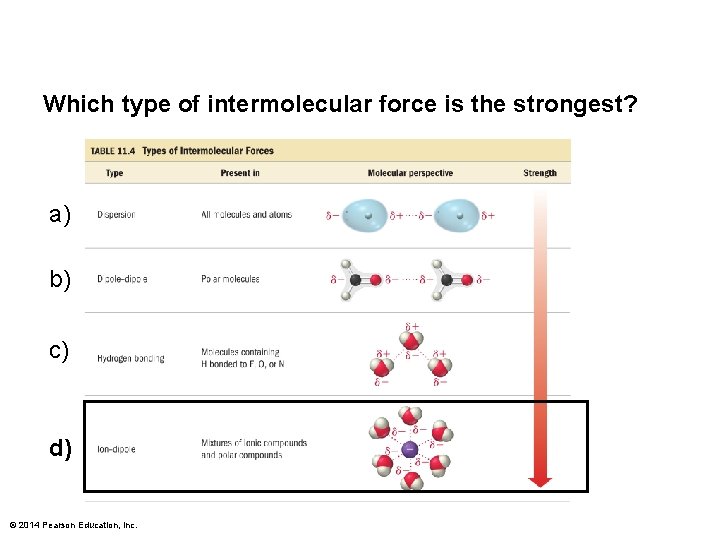
Which type of intermolecular force is the strongest? a) b) c) d) © 2014 Pearson Education, Inc.

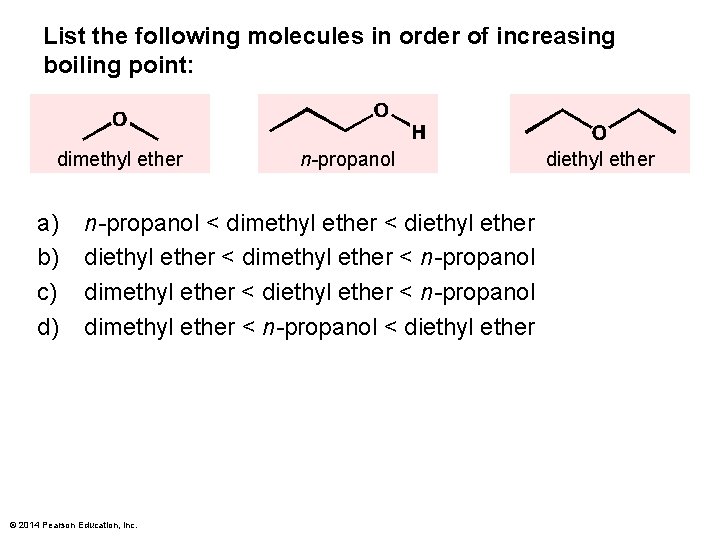
List the following molecules in order of increasing boiling point: dimethyl ether a) b) c) d) n-propanol < dimethyl ether < diethyl ether < dimethyl ether < n-propanol dimethyl ether < diethyl ether < n-propanol dimethyl ether < n-propanol < diethyl ether © 2014 Pearson Education, Inc. diethyl ether

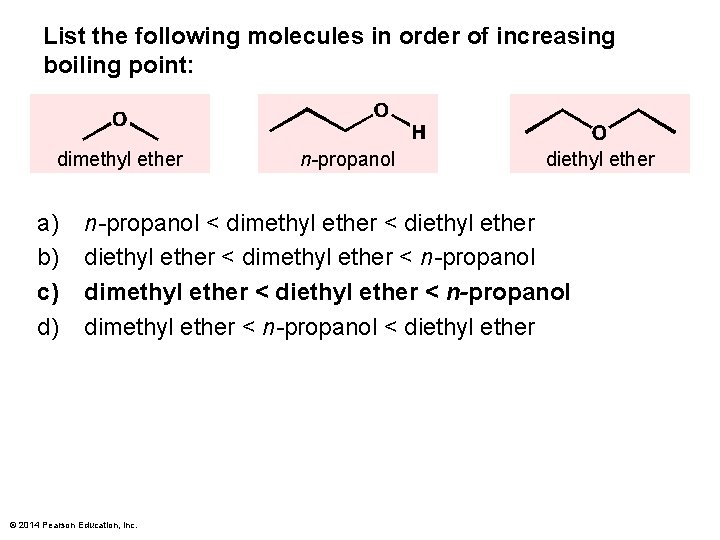
List the following molecules in order of increasing boiling point: dimethyl ether a) b) c) d) n-propanol diethyl ether n-propanol < dimethyl ether < diethyl ether < dimethyl ether < n-propanol dimethyl ether < diethyl ether < n-propanol dimethyl ether < n-propanol < diethyl ether © 2014 Pearson Education, Inc.

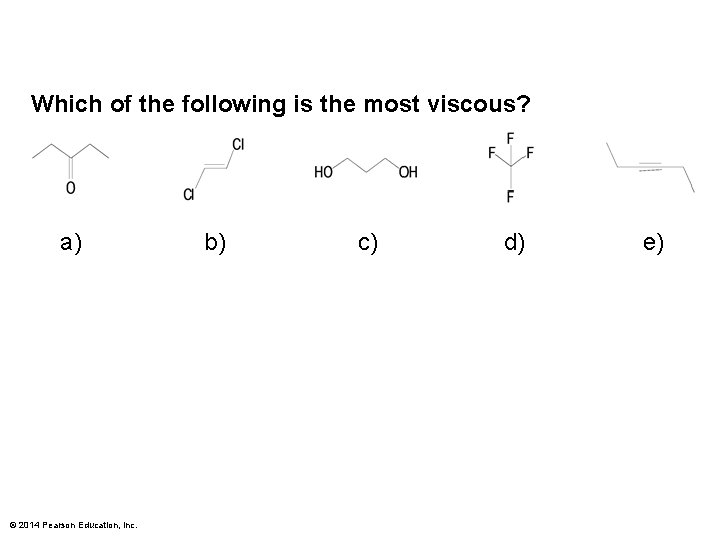
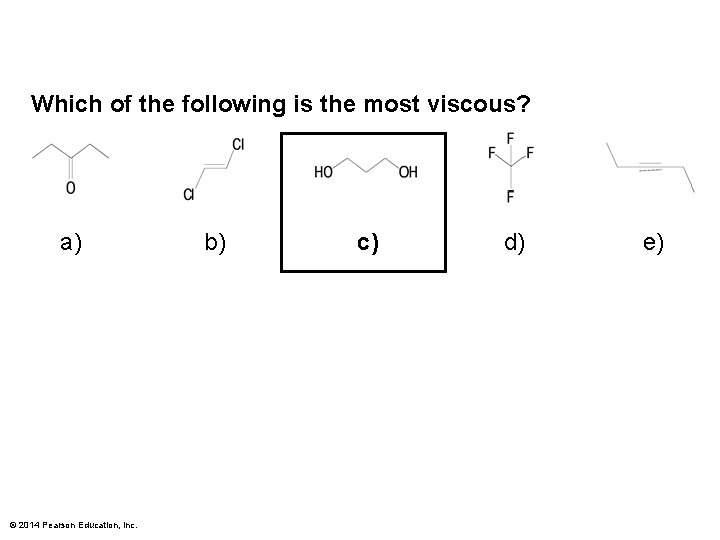
Which of the following is the most viscous? a) © 2014 Pearson Education, Inc. b) c) d) e)

Which of the following is the most viscous? a) © 2014 Pearson Education, Inc. b) c) d) e)

A graph is prepared from the natural log of pressure versus the inverse of the temperature. The slope of the resulting line is – 2996 K. What is the enthalpy of vaporization? a) b) c) d) 24. 91 k. J/mol − 24. 91 k. J/mol 245. 9 k. J/mol – 245. 9 k. J/mol © 2014 Pearson Education, Inc.

A graph is prepared from the natural log of pressure versus the inverse of the temperature. The slope of the resulting line is – 2996 K. What is the enthalpy of vaporization? a) b) c) d) 24. 91 k. J/mol − 24. 91 k. J/mol 245. 9 k. J/mol – 245. 9 k. J/mol © 2014 Pearson Education, Inc.

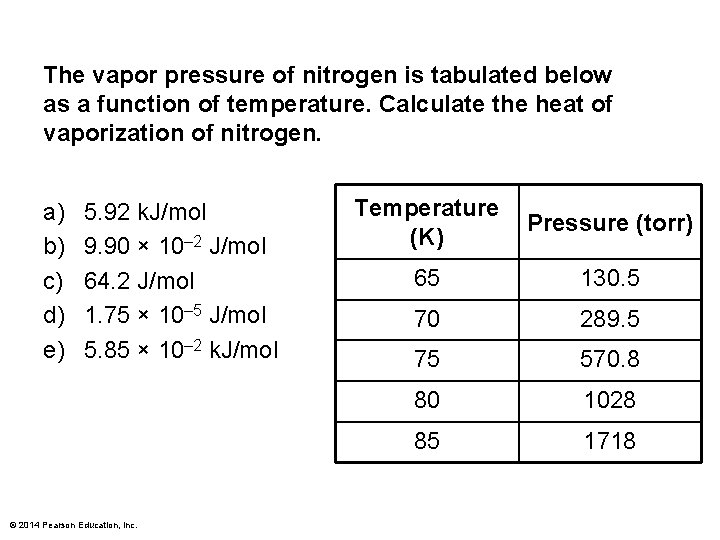
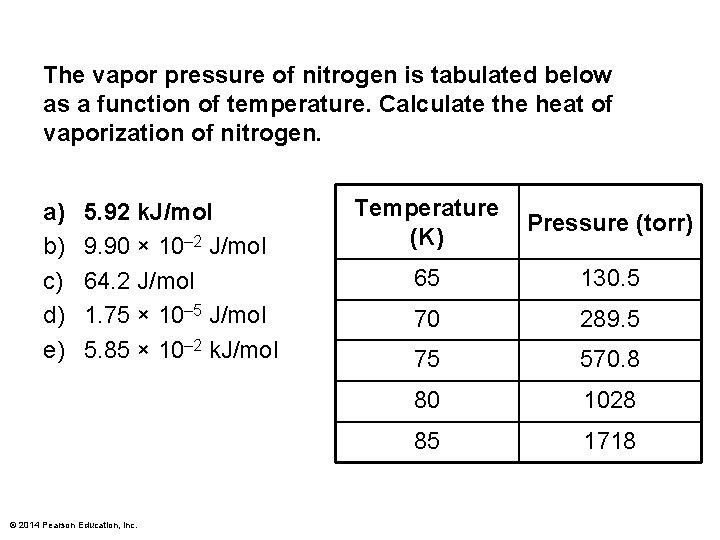
The vapor pressure of nitrogen is tabulated below as a function of temperature. Calculate the heat of vaporization of nitrogen. a) b) c) d) e) 5. 92 k. J/mol 9. 90 × 10– 2 J/mol 64. 2 J/mol 1. 75 × 10– 5 J/mol 5. 85 × 10– 2 k. J/mol © 2014 Pearson Education, Inc. Temperature (K) Pressure (torr) 65 130. 5 70 289. 5 75 570. 8 80 1028 85 1718

The vapor pressure of nitrogen is tabulated below as a function of temperature. Calculate the heat of vaporization of nitrogen. a) b) c) d) e) 5. 92 k. J/mol 9. 90 × 10– 2 J/mol 64. 2 J/mol 1. 75 × 10– 5 J/mol 5. 85 × 10– 2 k. J/mol © 2014 Pearson Education, Inc. Temperature (K) Pressure (torr) 65 130. 5 70 289. 5 75 570. 8 80 1028 85 1718

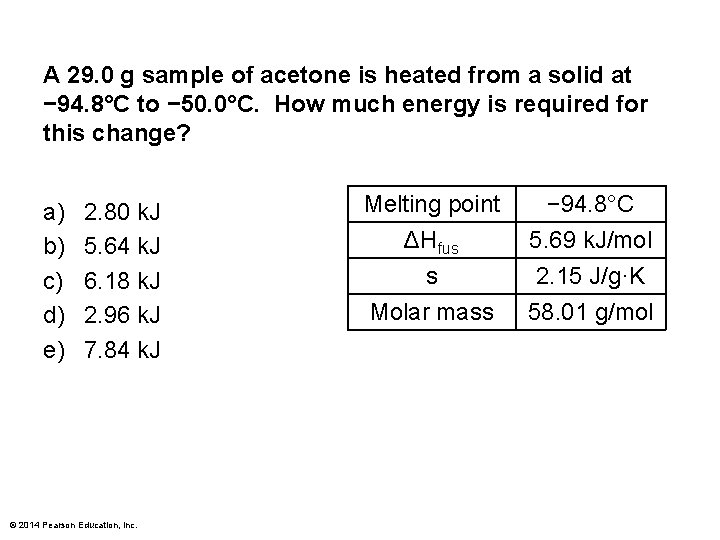
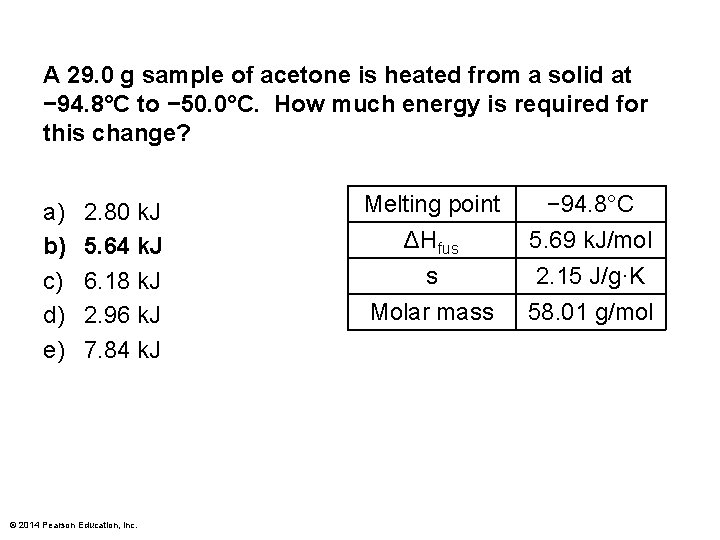
A 29. 0 g sample of acetone is heated from a solid at − 94. 8°C to − 50. 0°C. How much energy is required for this change? a) b) c) d) e) 2. 80 k. J 5. 64 k. J 6. 18 k. J 2. 96 k. J 7. 84 k. J © 2014 Pearson Education, Inc. Melting point ΔHfus s Molar mass − 94. 8°C 5. 69 k. J/mol 2. 15 J/g·K 58. 01 g/mol

A 29. 0 g sample of acetone is heated from a solid at − 94. 8°C to − 50. 0°C. How much energy is required for this change? a) b) c) d) e) 2. 80 k. J 5. 64 k. J 6. 18 k. J 2. 96 k. J 7. 84 k. J © 2014 Pearson Education, Inc. Melting point ΔHfus s Molar mass − 94. 8°C 5. 69 k. J/mol 2. 15 J/g·K 58. 01 g/mol

How much energy is required to vaporize 32. 9 g of ethanol at its boiling point, if its Hvap is 40. 7 k. J/mol? a) b) c) d) e) 28. 9 k. J 3. 83 k. J 6. 30 k. J 17. 6 k. J 7. 60 k. J © 2014 Pearson Education, Inc.

How much energy is required to vaporize 32. 9 g of ethanol at its boiling point, if its Hvap is 40. 7 k. J/mol? a) b) c) d) e) 28. 9 k. J 3. 83 k. J 6. 30 k. J 17. 6 k. J 7. 60 k. J © 2014 Pearson Education, Inc.

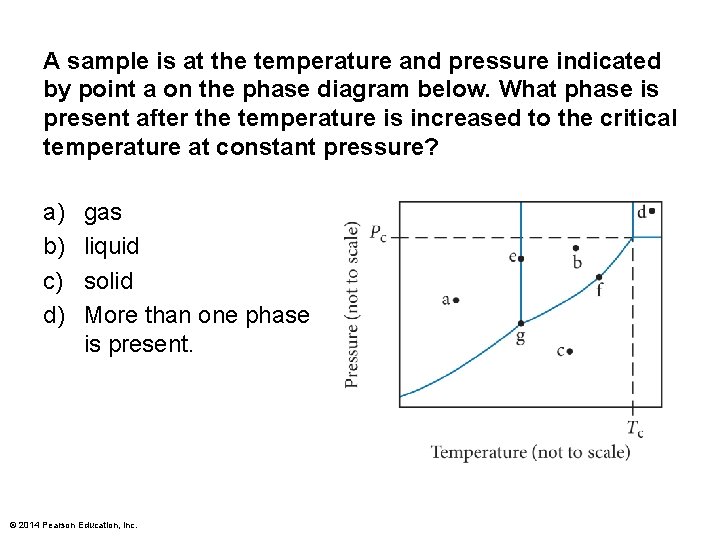
A sample is at the temperature and pressure indicated by point a on the phase diagram below. What phase is present after the temperature is increased to the critical temperature at constant pressure? a) b) c) d) gas liquid solid More than one phase is present. © 2014 Pearson Education, Inc.

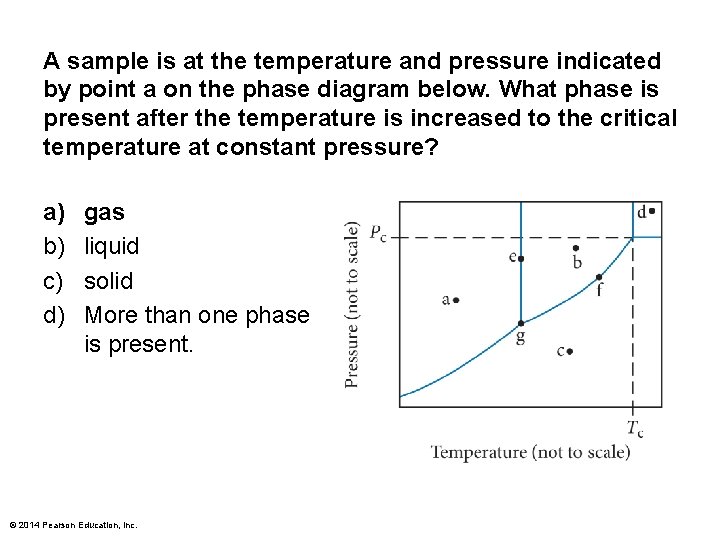
A sample is at the temperature and pressure indicated by point a on the phase diagram below. What phase is present after the temperature is increased to the critical temperature at constant pressure? a) b) c) d) gas liquid solid More than one phase is present. © 2014 Pearson Education, Inc.

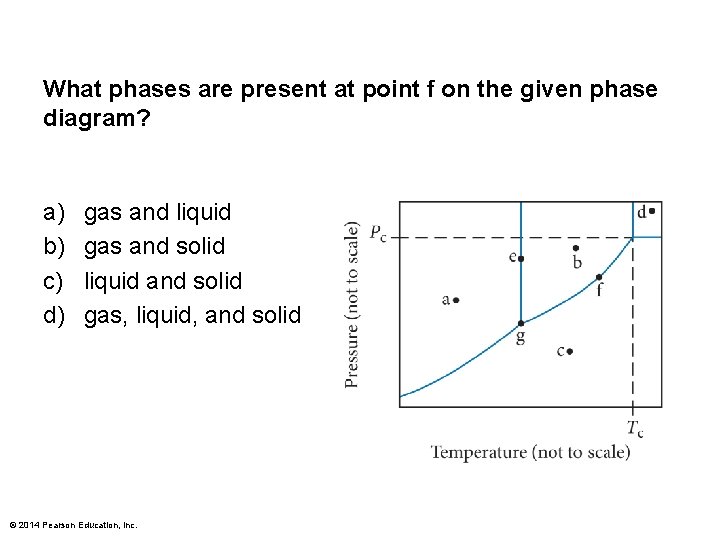
What phases are present at point f on the given phase diagram? a) b) c) d) gas and liquid gas and solid liquid and solid gas, liquid, and solid © 2014 Pearson Education, Inc.

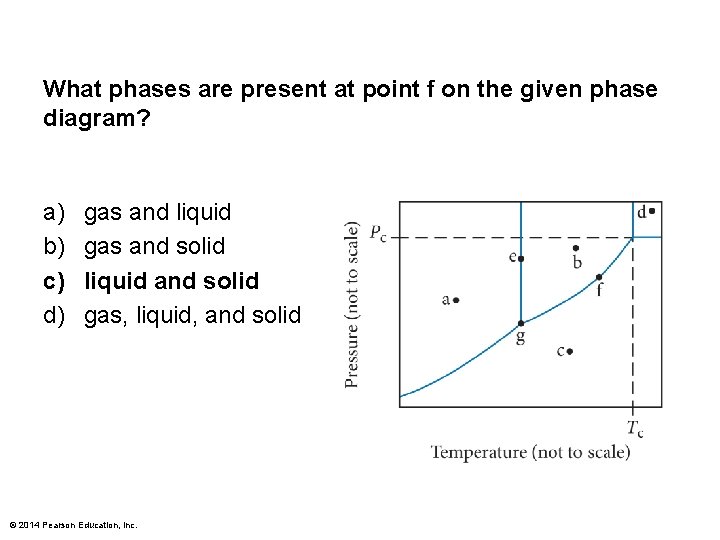
What phases are present at point f on the given phase diagram? a) b) c) d) gas and liquid gas and solid liquid and solid gas, liquid, and solid © 2014 Pearson Education, Inc.

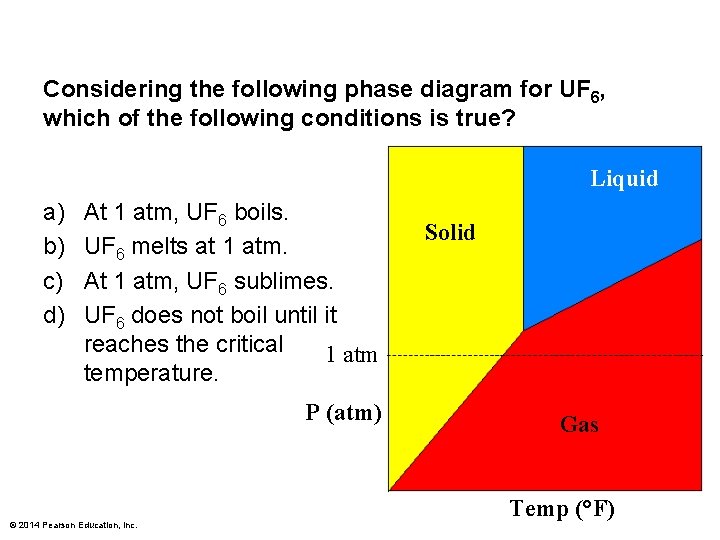
Considering the following phase diagram for UF 6, which of the following conditions is true? Liquid a) b) c) d) At 1 atm, UF 6 boils. UF 6 melts at 1 atm. At 1 atm, UF 6 sublimes. UF 6 does not boil until it reaches the critical 1 atm temperature. P (atm) © 2014 Pearson Education, Inc. Solid Gas Temp (°F)

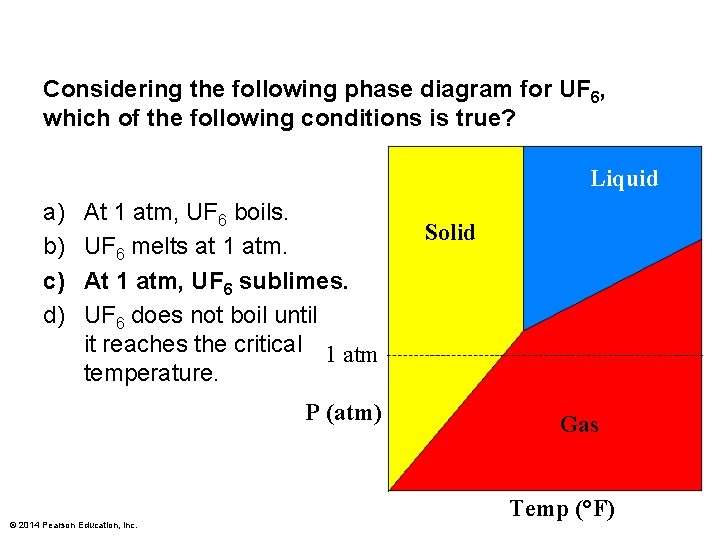
Considering the following phase diagram for UF 6, which of the following conditions is true? Liquid a) b) c) d) At 1 atm, UF 6 boils. UF 6 melts at 1 atm. At 1 atm, UF 6 sublimes. UF 6 does not boil until it reaches the critical 1 atm temperature. P (atm) © 2014 Pearson Education, Inc. Solid Gas Temp (°F)

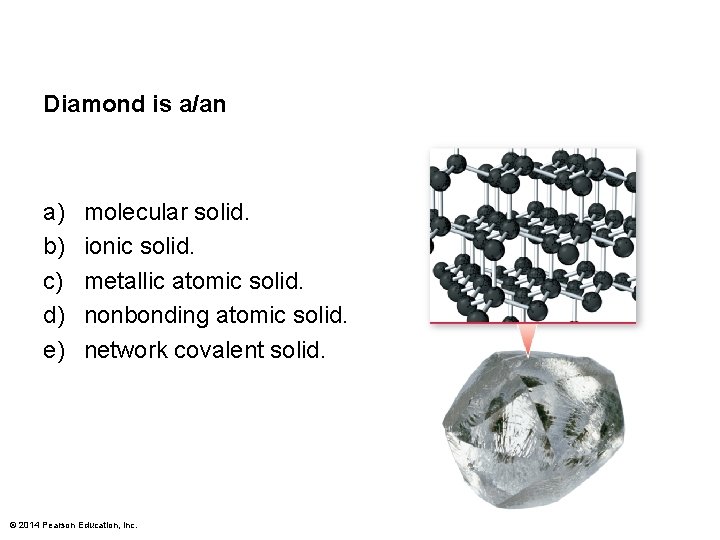
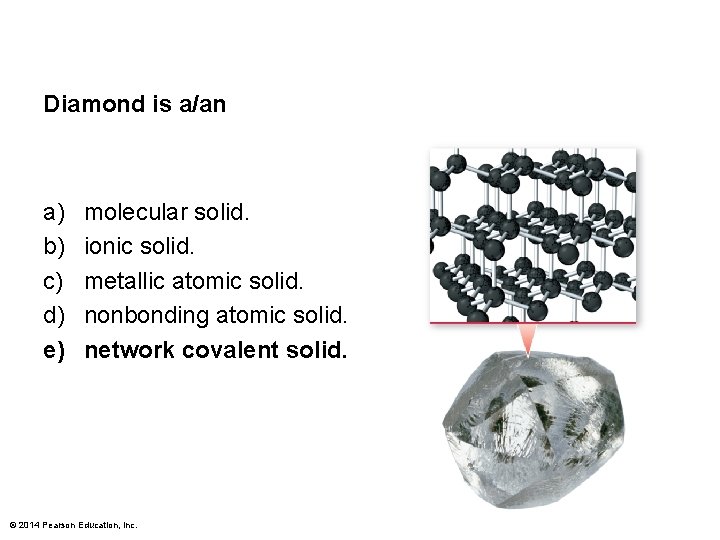
Diamond is a/an a) b) c) d) e) molecular solid. ionic solid. metallic atomic solid. nonbonding atomic solid. network covalent solid. © 2014 Pearson Education, Inc.

Diamond is a/an a) b) c) d) e) molecular solid. ionic solid. metallic atomic solid. nonbonding atomic solid. network covalent solid. © 2014 Pearson Education, Inc.