Which ones will conduct Electrolytes vs NonElectrolytes Electrolyte






- Slides: 12

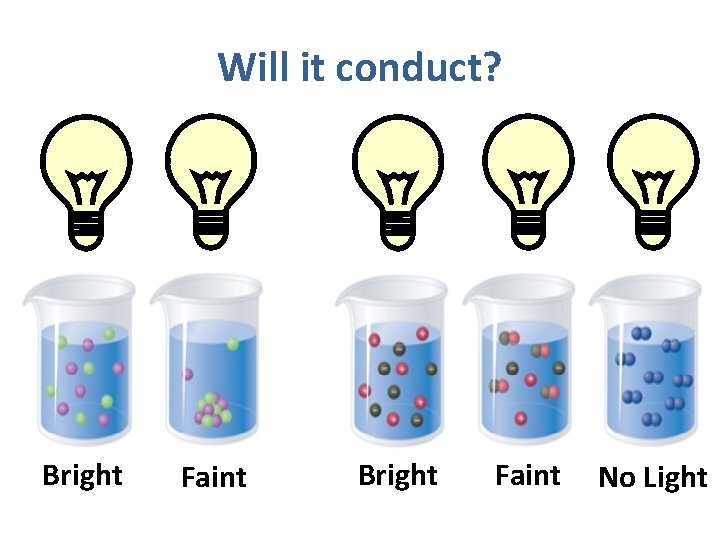
Which one(s) will conduct?

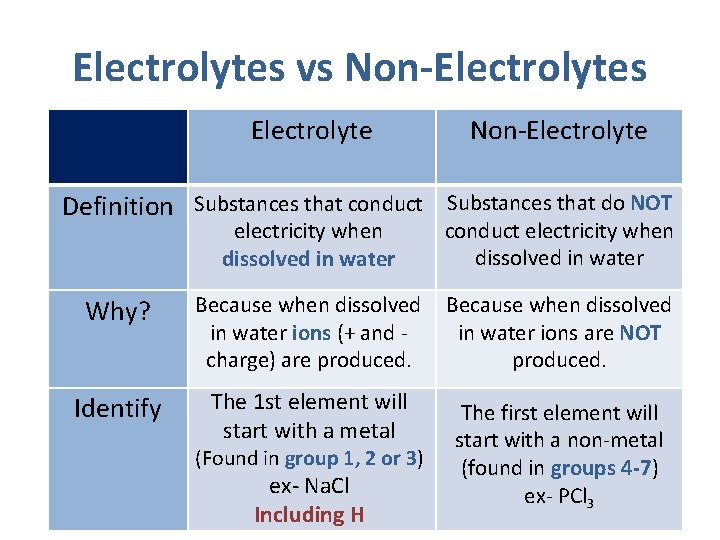
Electrolytes vs Non-Electrolytes Electrolyte Non-Electrolyte Definition Substances that conduct Substances that do NOT electricity when dissolved in water conduct electricity when dissolved in water Why? Because when dissolved in water ions (+ and charge) are produced. Because when dissolved in water ions are NOT produced. Identify The 1 st element will start with a metal The first element will start with a non-metal (found in groups 4 -7) ex- PCl 3 (Found in group 1, 2 or 3) ex- Na. Cl Including H

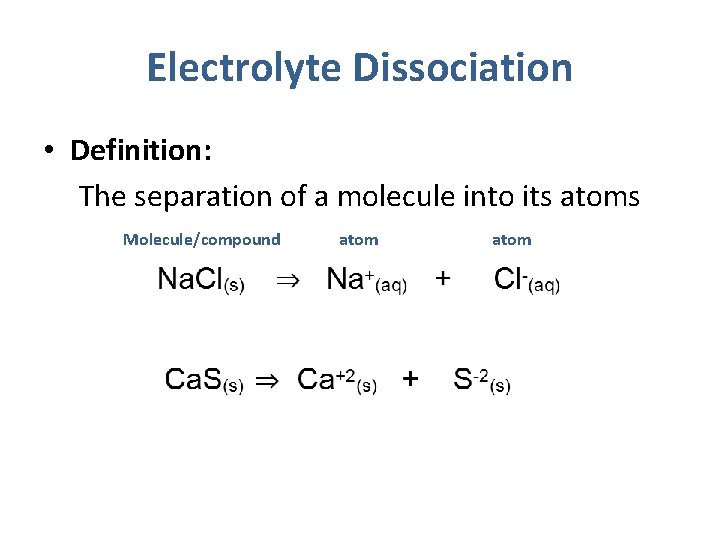
Electrolyte Dissociation • Definition: The separation of a molecule into its atoms Molecule/compound atom


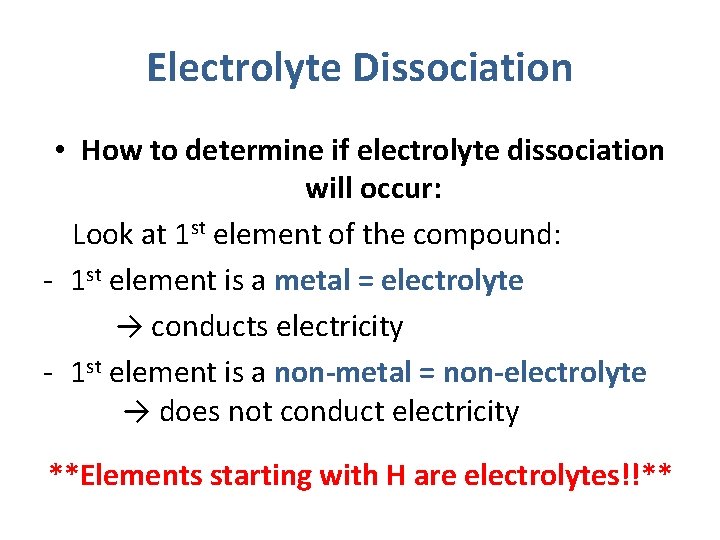
Electrolyte Dissociation • How to determine if electrolyte dissociation will occur: Look at 1 st element of the compound: - 1 st element is a metal = electrolyte → conducts electricity - 1 st element is a non-metal = non-electrolyte → does not conduct electricity **Elements starting with H are electrolytes!!**

Practice Which ones will conduct electricity? Ca. Cl 2 → E H 2 SO 4 → E P 2 S 3 → NE KOH → E SCl 2 → NE CCl 4 → NE

Will it conduct? Bright Faint No Light

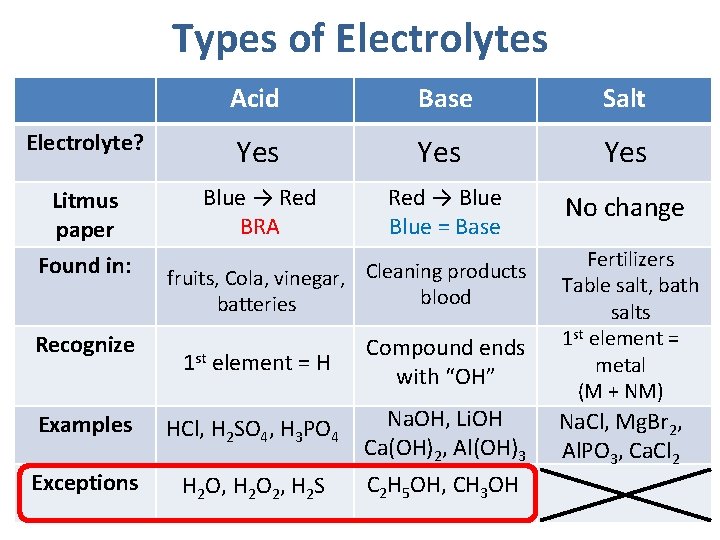
Types of Electrolytes Acid Base Salt Electrolyte? Yes Yes Litmus paper Blue → Red BRA Red → Blue = Base No change Found in: Recognize fruits, Cola, vinegar, Cleaning products blood batteries 1 st element = H Compound ends with “OH” Examples HCl, H 2 SO 4, H 3 PO 4 Na. OH, Li. OH Ca(OH)2, Al(OH)3 Exceptions H 2 O, H 2 O 2, H 2 S C 2 H 5 OH, CH 3 OH Fertilizers Table salt, bath salts 1 st element = metal (M + NM) Na. Cl, Mg. Br 2, Al. PO 3, Ca. Cl 2

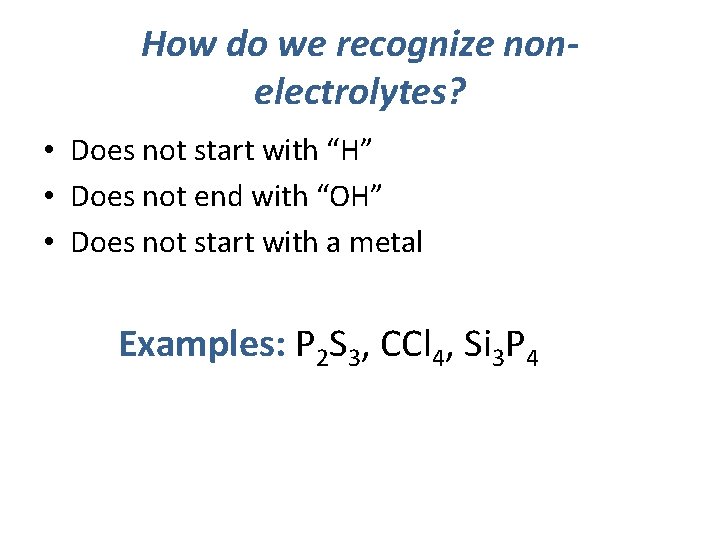
How do we recognize nonelectrolytes? • Does not start with “H” • Does not end with “OH” • Does not start with a metal Examples: P 2 S 3, CCl 4, Si 3 P 4

Past Exam Questions 1. You have a sample of sodium hydroxide (Na. OH), which is a white solid. You want to show in the laboratory that this sample is a base. Under which condition will this sample manifest its basic properties? A) On condition that it is dissolved in water B) On condition that there is sufficient quantity of it C) On condition that it is sufficiently compressed D) On condition that it is in powder form

Past Exam Questions 2. The following four compounds are to be mixed (separately) with water: C 6 H 12 O 6 Mg. SO 4 C 2 H 5 OH KOH Which two of these compounds will produce an electrolytic solution when mixed with water? A) C 6 H 12 O 6 and Mg. SO 4 B) Mg. SO 4 and KOH C) C 6 H 12 O 6 and C 2 H 5 OH D) C 2 H 5 OH and KOH

Past Exam Questions 3. Four chemical substances are given below. 1. H 2 SO 4 2. Ca(OH)2 3. Mg. Cl 2 4. C 2 H 5 OH Which of these substances is a base? A) Substance 1 B) Substance 2 C) Substance 3 D) Substance 4