When a chemical bond is broken energy is

When a chemical bond is broken, energy is A. ) absorbed, only B. ) released, only C. ) both absorbed and released D. ) neither absorbed nor released
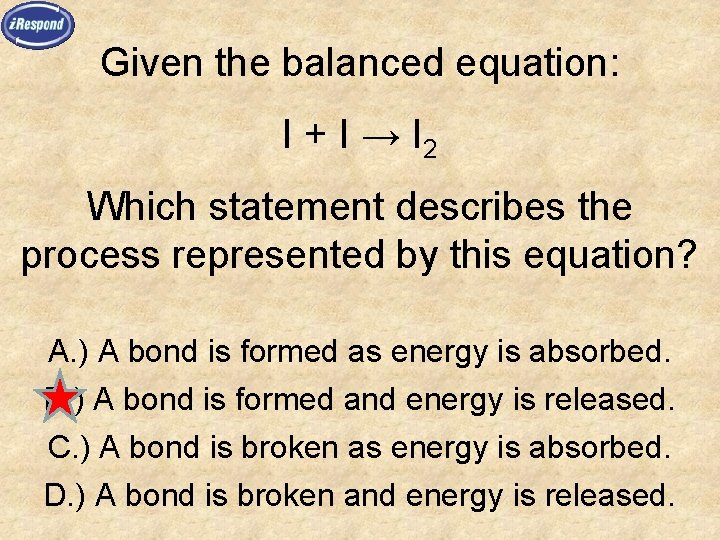
Given the balanced equation: I + I → I 2 Which statement describes the process represented by this equation? A. ) A bond is formed as energy is absorbed. B. ) A bond is formed and energy is released. C. ) A bond is broken as energy is absorbed. D. ) A bond is broken and energy is released.

Which atom will form an ionic bond with a Br atom? Ionic Bond: Metal and a Nonmetal Bromine is a nonmetal A. ) N B. ) Li C. ) O D. ) C

When ionic bonds are formed, metallic atoms tend to A. ) lose electrons and become negative ions B. ) lose electrons and become positive ions C. ) gain electrons and become negative ions D. ) gain electrons and become positive ions
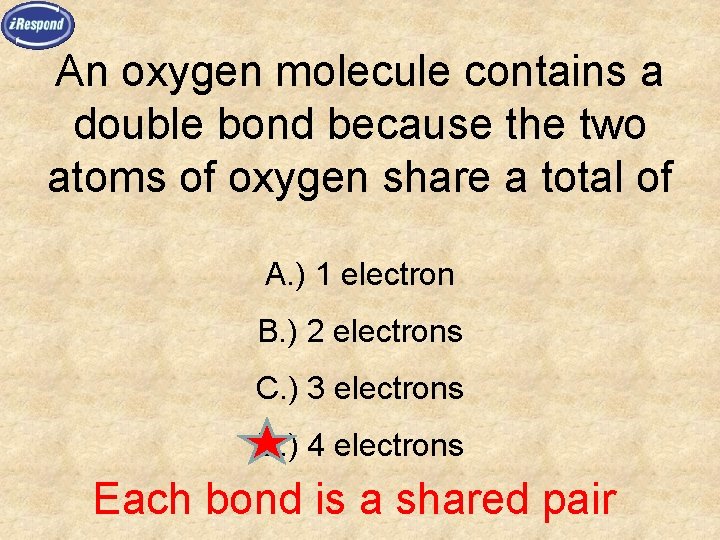
An oxygen molecule contains a double bond because the two atoms of oxygen share a total of A. ) 1 electron B. ) 2 electrons C. ) 3 electrons D. ) 4 electrons Each bond is a shared pair
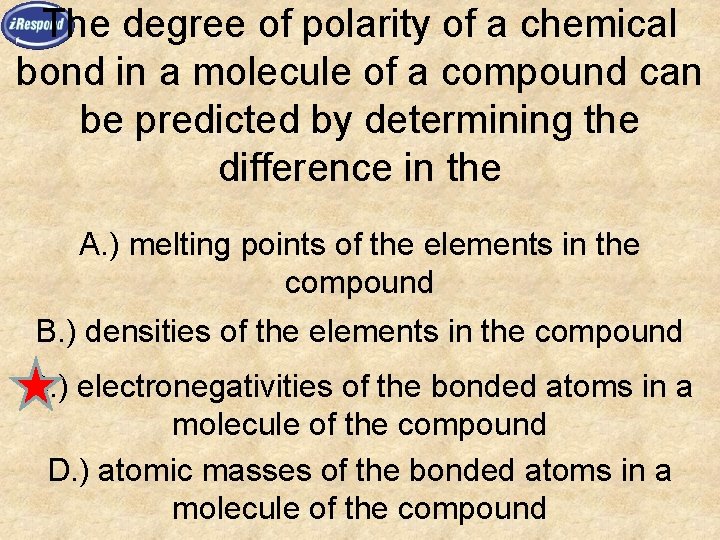
The degree of polarity of a chemical bond in a molecule of a compound can be predicted by determining the difference in the A. ) melting points of the elements in the compound B. ) densities of the elements in the compound C. ) electronegativities of the bonded atoms in a molecule of the compound D. ) atomic masses of the bonded atoms in a molecule of the compound

In a nonpolar covalent bond, electrons are A. ) located in a mobile "sea" shared by many ions B. ) transferred from one atom to another C. ) shared equally to two atoms D. ) shared unequally by two atoms

Based on electronegativity values, which type of elements tends to have the greatest attraction for electrons in a bond? A. ) metals B. ) metalloids C. ) nonmetals Fluorine most D. ) noble gases

The ability of carbon to attract electrons is A. ) greater than that of nitrogen, but less than that of oxygen B. ) less than that of nitrogen, but greater than that of oxygen C. ) greater than that of nitrogen and oxygen D. ) less than that of nitrogen and oxygen Farther from fluorine

Which bond is most polar? Fluorine is most electronegative A. ) H-F B. ) H-Cl C. ) H-Br D. ) H-I
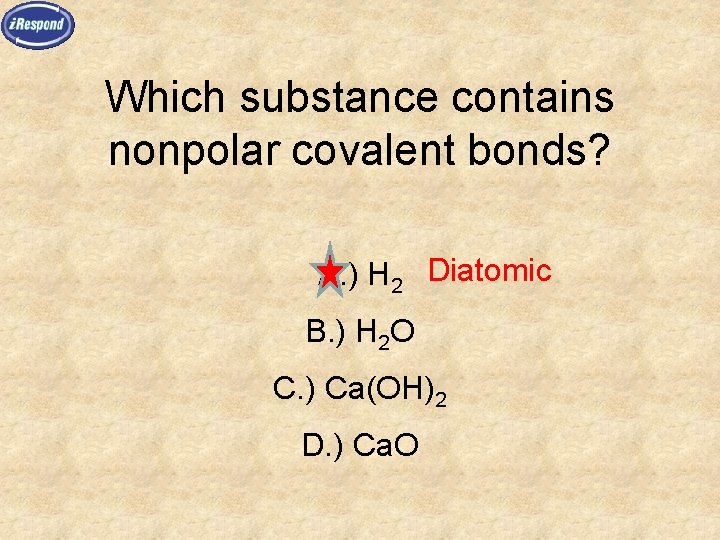
Which substance contains nonpolar covalent bonds? A. ) H 2 Diatomic B. ) H 2 O C. ) Ca(OH)2 D. ) Ca. O
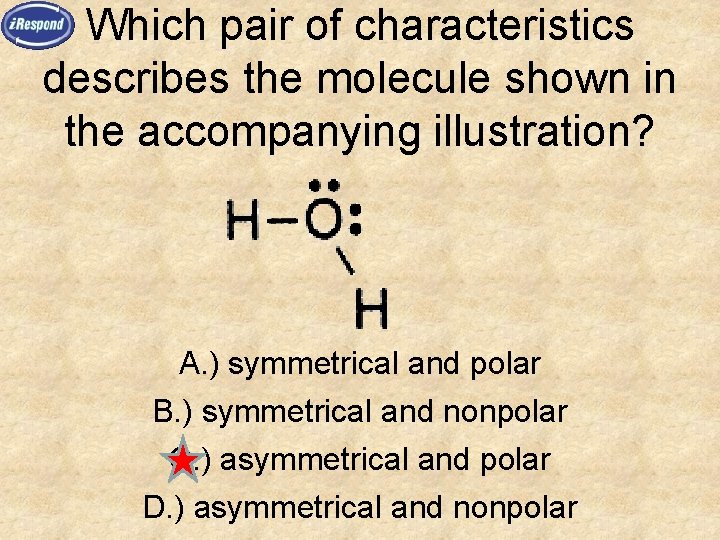
Which pair of characteristics describes the molecule shown in the accompanying illustration? A. ) symmetrical and polar B. ) symmetrical and nonpolar C. ) asymmetrical and polar D. ) asymmetrical and nonpolar
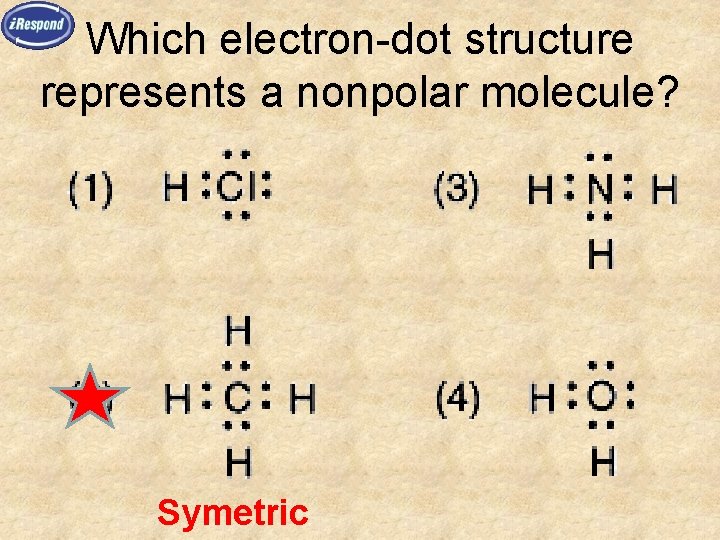
Which electron-dot structure represents a nonpolar molecule? Symetric
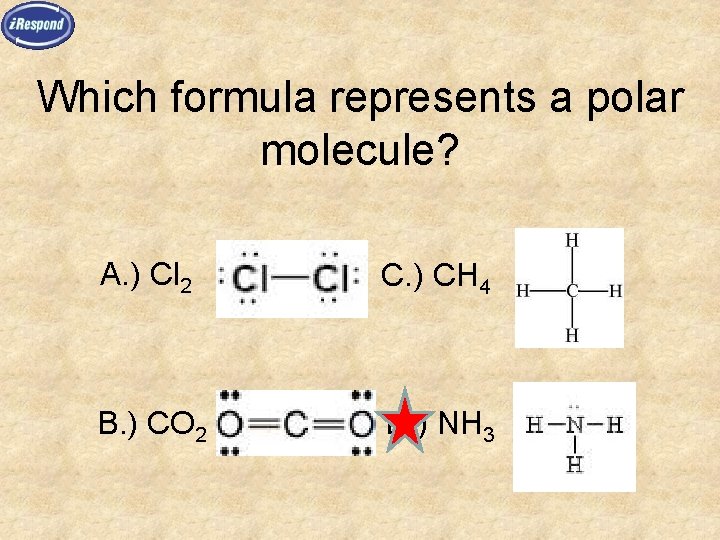
Which formula represents a polar molecule? A. ) Cl 2 C. ) CH 4 B. ) CO 2 D. ) NH 3

Which characteristic is a property of molecular substances? A. ) good heat conductivity B. ) good electrical conductivity C. ) low melting point D. ) high melting point
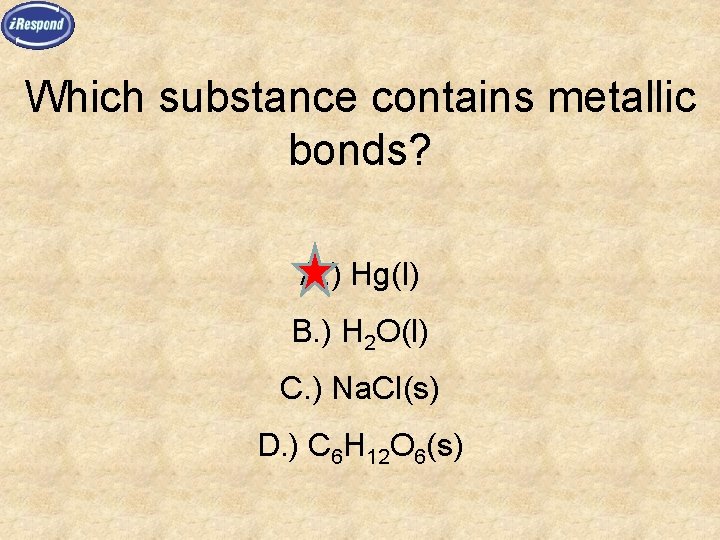
Which substance contains metallic bonds? A. ) Hg(l) B. ) H 2 O(l) C. ) Na. Cl(s) D. ) C 6 H 12 O 6(s)

Conductivity in a metal results from the metal atoms having A. ) high electronegativity B. ) high ionization energy C. ) highly mobile protons in the nucleus D. ) highly mobile electrons in the valence shell

Which statement best describes the substance that results when electrons are transferred from a metal to a nonmetal? A. ) It contains ionic bonds and has a low melting point. B. ) It contains ionic bonds and has a high melting point. C. ) It contains covalent bonds and has a low melting point. D. ) It contains covalent bonds and has a high melting point.

In which compound do atoms form bonds by sharing electrons? Sharing = Covalent = 2 nonmetals A. ) H 2 O B. ) Na 2 O C. ) Ca. O D. ) Mg. O

When a reaction occurs between atoms with ground-state electron configurations of 1 s 22 s 1 and 1 s 22 p 5, the bond formed is mainly Boron (metal) + Fluorine (nonmetal) A. ) polar covalent B. ) nonpolar covalent C. ) metallic D. ) ionic

The bonds in the compound Mg. SO 4 can be described as Contains a polyatomic ion. Covalent between the sulfur and oxygen in sulfate and ionic between the magnesium ion and the sulfate ion A. ) ionic, only B. ) covalent, only C. ) both ionic and covalent D. ) neither ionic nor covalent

Metallic bonding occurs between metal atoms that have A. ) full valence orbitals and low ionization energies B. ) full valence orbitals and high ionization energies C. ) vacant valence orbitals and low ionization energies D. ) vacant valence orbitals and high ionization energies
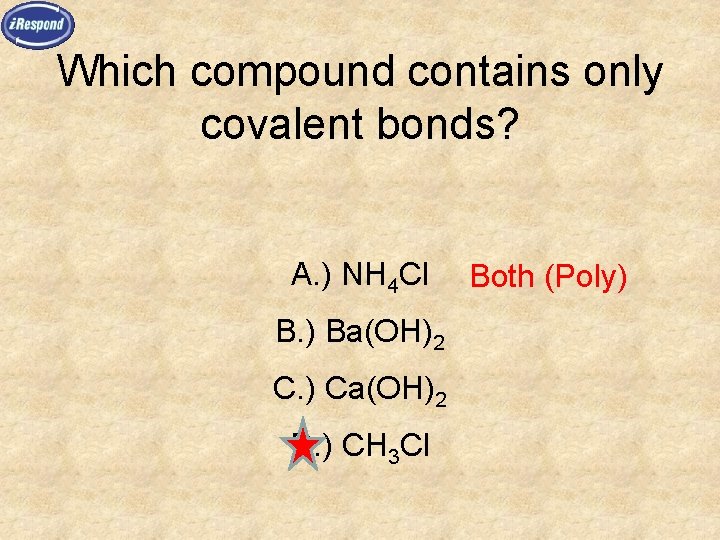
Which compound contains only covalent bonds? A. ) NH 4 Cl B. ) Ba(OH)2 C. ) Ca(OH)2 D. ) CH 3 Cl Both (Poly)

In which compound have electrons been transferred to the oxygen atom? Transfer = Ionic = metal + nonmetal A. ) CO 2 B. ) NO 2 C. ) N 2 O D. ) Na 2 O
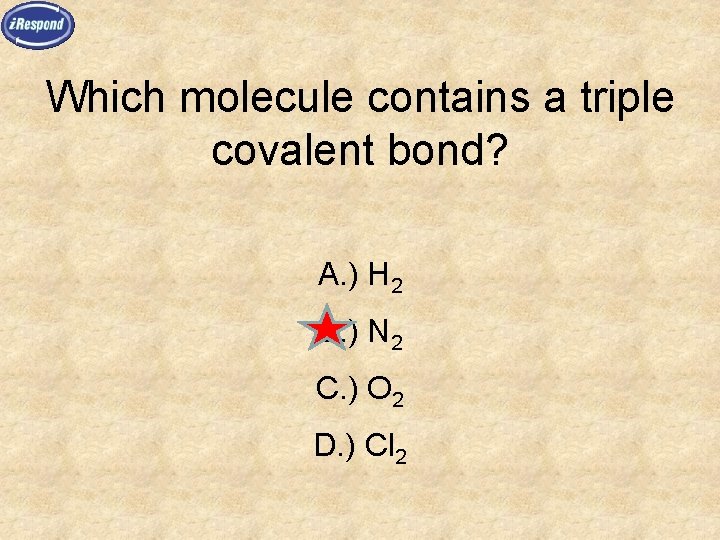
Which molecule contains a triple covalent bond? A. ) H 2 B. ) N 2 C. ) O 2 D. ) Cl 2

Which type of substance is soft, has a low melting point, and is a poor conductor of heat and electricity? A. ) network solid B. ) molecular solid C. ) metallic solid D. ) ionic solid
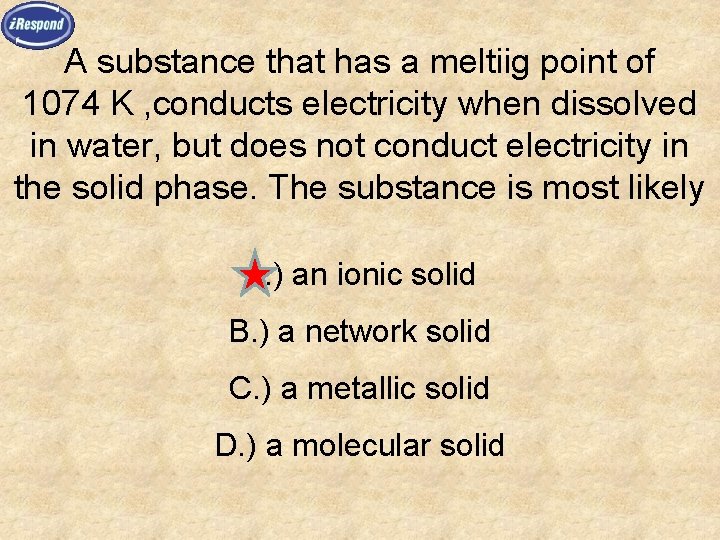
A substance that has a meltiig point of 1074 K , conducts electricity when dissolved in water, but does not conduct electricity in the solid phase. The substance is most likely A. ) an ionic solid B. ) a network solid C. ) a metallic solid D. ) a molecular solid
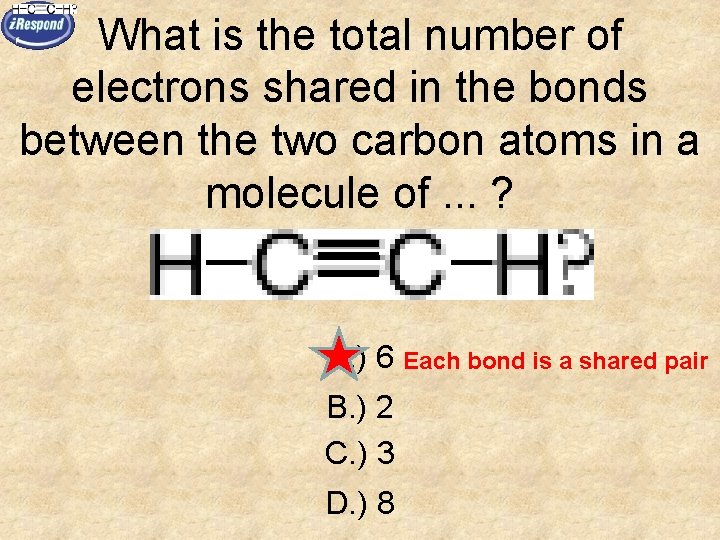
What is the total number of electrons shared in the bonds between the two carbon atoms in a molecule of. . . ? A. ) 6 Each bond is a shared pair B. ) 2 C. ) 3 D. ) 8
The bond between hydrogen and oxygen in a water molecule is classified as A. ) ionic and nonpolar B. ) ionic and polar C. ) covalent and nonpolar D. ) covalent and polar Metal + nonmetal = ionic Nonmetal + nonmetal = polar covalent (unless diatomic)

Which substance is correctly paired with its type of bonding? A. ) Na. Br, nonpolar covalent Ionic B. ) HCl, nonpolar covalent Polar covalent C. ) NH 3, polar covalent D. ) Br 2, polar covalent Nonpolar covalent
Which type of molecule is CF 4? A. ) polar, with a symmetrical distribution of charge B. ) polar, with an asymmetrical distribution of charge C. ) nonpolar, with a symmetrical distribution of charge D. ) nonpolar, with an asymmetrical distribution of charge
Which formula represents a molecular substance? Molecular = Covalent = 2 nonmetals A. ) Ca. O B. ) CO C. ) Li 2 O D. ) Al 2 O 3

What is the formula for the compound formed by calcium ions and chloride ions? Ca+2 Cl-1 A. ) Ca. Cl B. ) Ca 2 Cl C. ) Ca. Cl 3 D. ) Ca. Cl 2

What is the formula for zinc fluoride? Zn+2 F-1 A. ) Zn. F B. ) Zn. F 2 C. ) Zn 2 F D. ) Zn 2 F 3
- Slides: 34