Whats the difference between s l g Steam
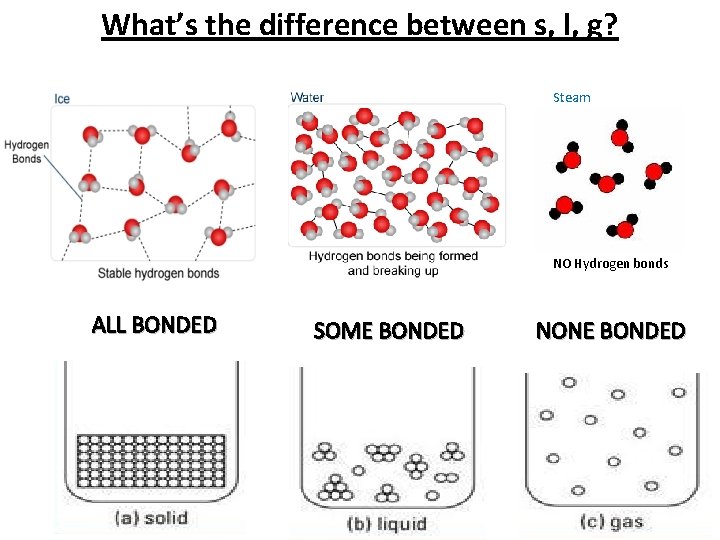
What’s the difference between s, l, g? Steam NO Hydrogen bonds ALL BONDED SOME BONDED NONE BONDED
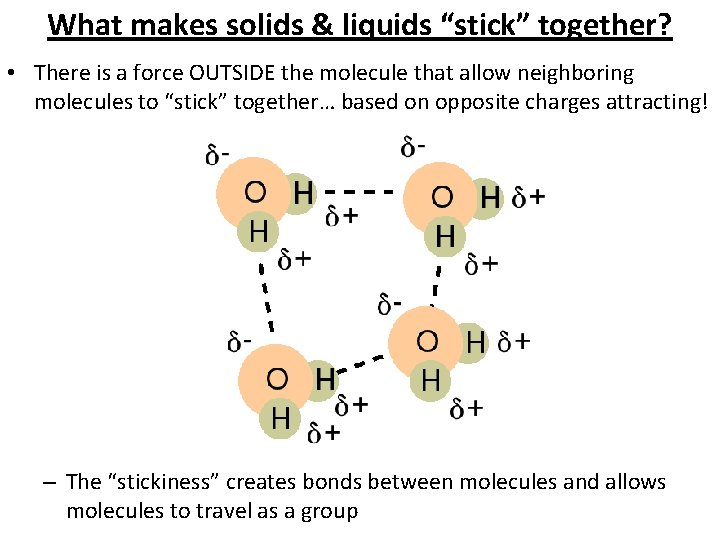
What makes solids & liquids “stick” together? • There is a force OUTSIDE the molecule that allow neighboring molecules to “stick” together… based on opposite charges attracting! – The “stickiness” creates bonds between molecules and allows molecules to travel as a group
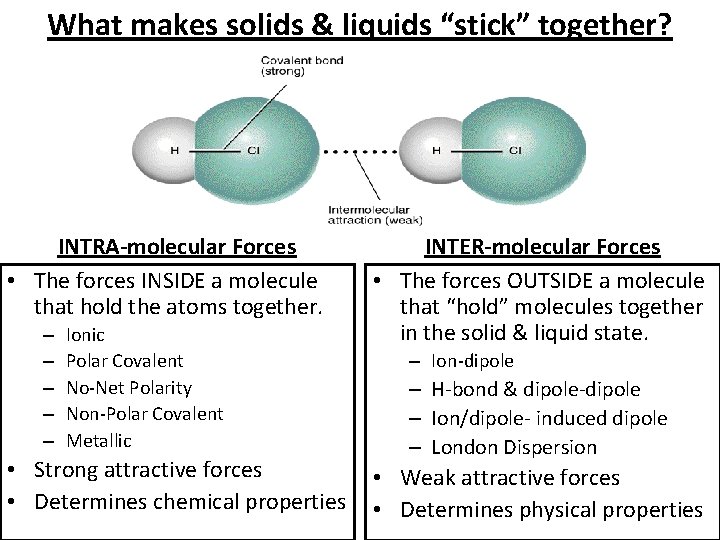
What makes solids & liquids “stick” together? INTRA-molecular Forces • The forces INSIDE a molecule that hold the atoms together. – – – Ionic Polar Covalent No-Net Polarity Non-Polar Covalent Metallic • Strong attractive forces • Determines chemical properties INTER-molecular Forces • The forces OUTSIDE a molecule that “hold” molecules together in the solid & liquid state. – Ion-dipole – H-bond & dipole-dipole – Ion/dipole- induced dipole – London Dispersion • Weak attractive forces • Determines physical properties
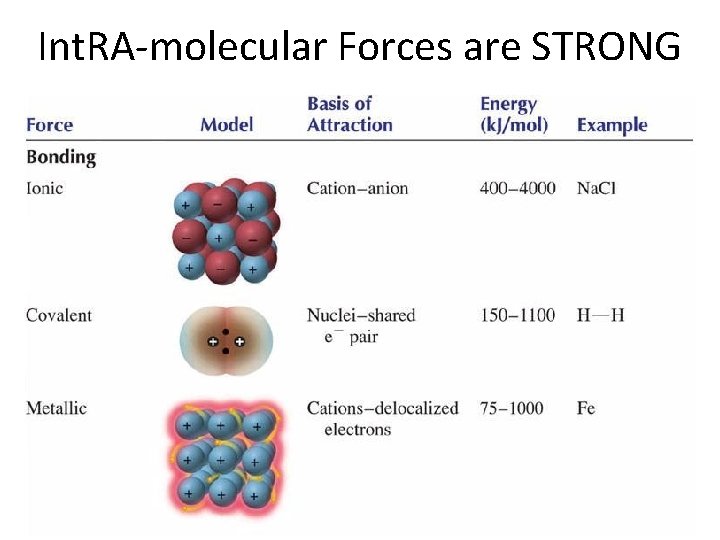
Int. RA-molecular Forces are STRONG
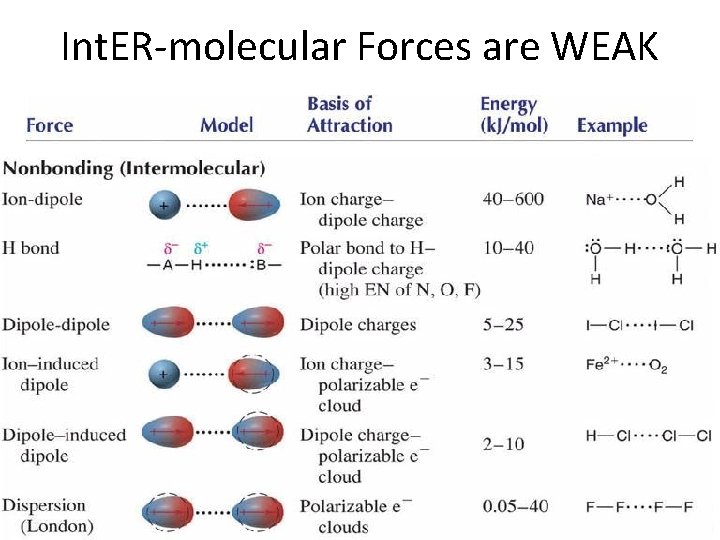
Int. ER-molecular Forces are WEAK
Strength of Inter-Molecular Forces • Depends on TYPE of inter-molecular force – Ion-dip > H-bond > dip-dip > ion-induced > dip-induced > dispersion • Magnitude of charge/dipole • Depends on SIZE of molecule – molar mass strength • Depend on SHAPE of molecule – Straight chain: contact strength – Branched chain: contact strength • Strength determines – Strength MP BP Solubility ΔH specific heat
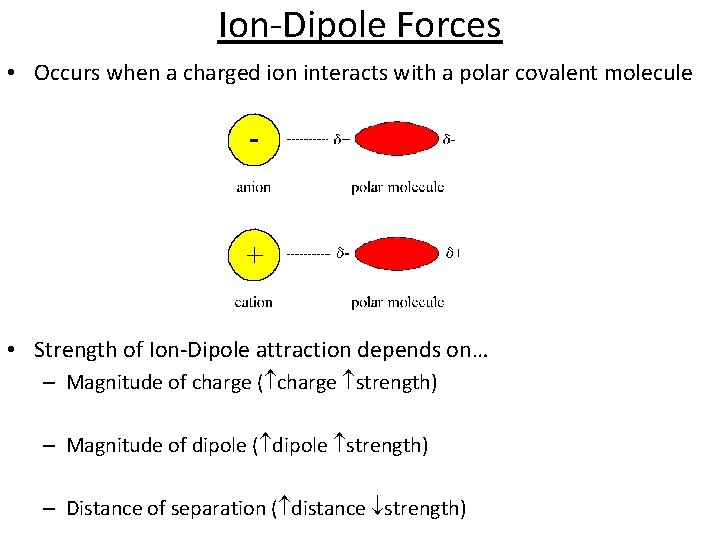
Ion-Dipole Forces • Occurs when a charged ion interacts with a polar covalent molecule • Strength of Ion-Dipole attraction depends on… – Magnitude of charge ( charge strength) – Magnitude of dipole ( dipole strength) – Distance of separation ( distance strength)
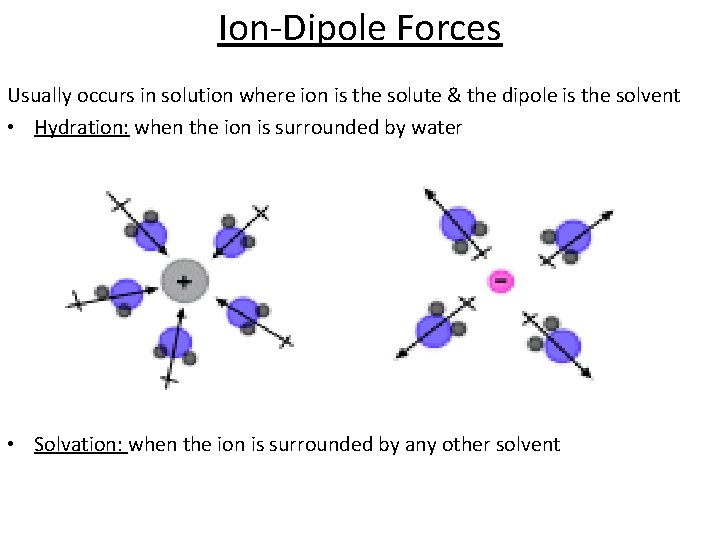
Ion-Dipole Forces Usually occurs in solution where ion is the solute & the dipole is the solvent • Hydration: when the ion is surrounded by water • Solvation: when the ion is surrounded by any other solvent
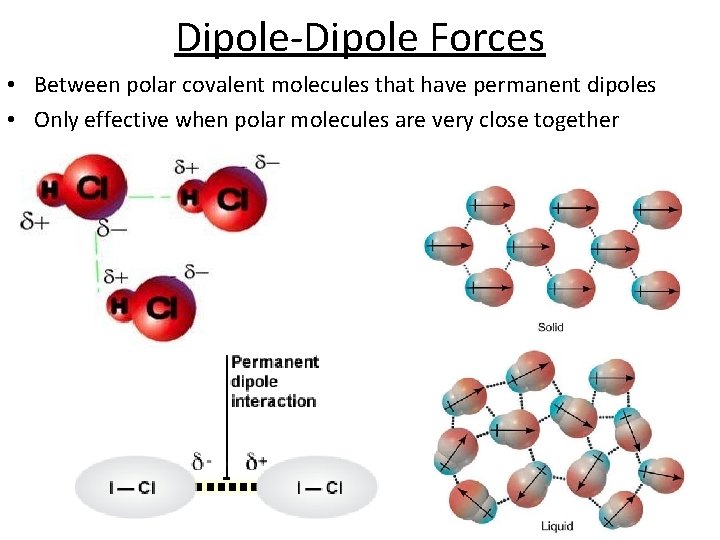
Dipole-Dipole Forces • Between polar covalent molecules that have permanent dipoles • Only effective when polar molecules are very close together
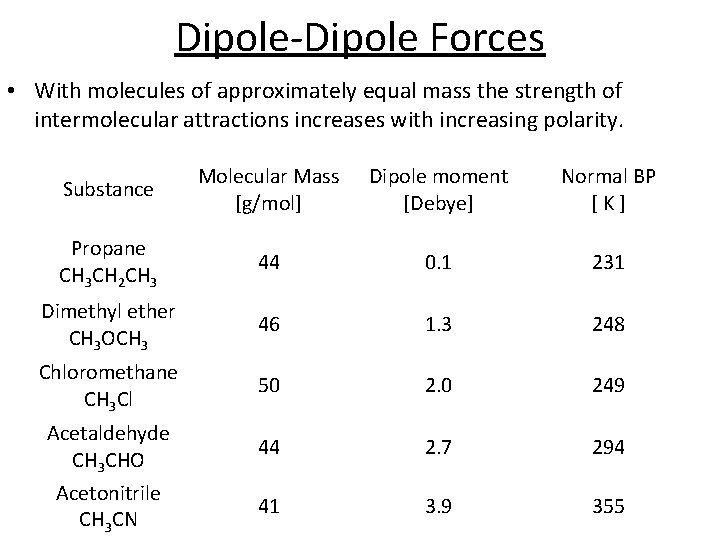
Dipole-Dipole Forces • With molecules of approximately equal mass the strength of intermolecular attractions increases with increasing polarity. Substance Molecular Mass [g/mol] Dipole moment [Debye] Normal BP [K] Propane CH 3 CH 2 CH 3 44 0. 1 231 Dimethyl ether CH 3 OCH 3 46 1. 3 248 Chloromethane CH 3 Cl 50 2. 0 249 44 2. 7 294 41 3. 9 355 Acetaldehyde CH 3 CHO Acetonitrile CH 3 CN
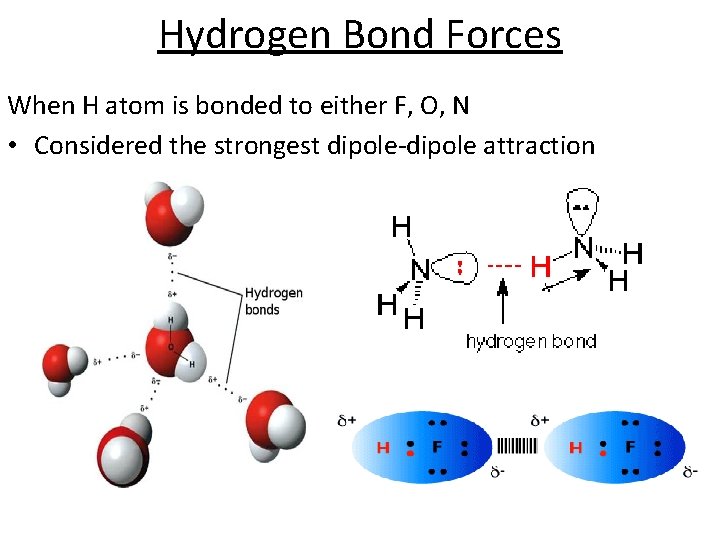
Hydrogen Bond Forces When H atom is bonded to either F, O, N • Considered the strongest dipole-dipole attraction
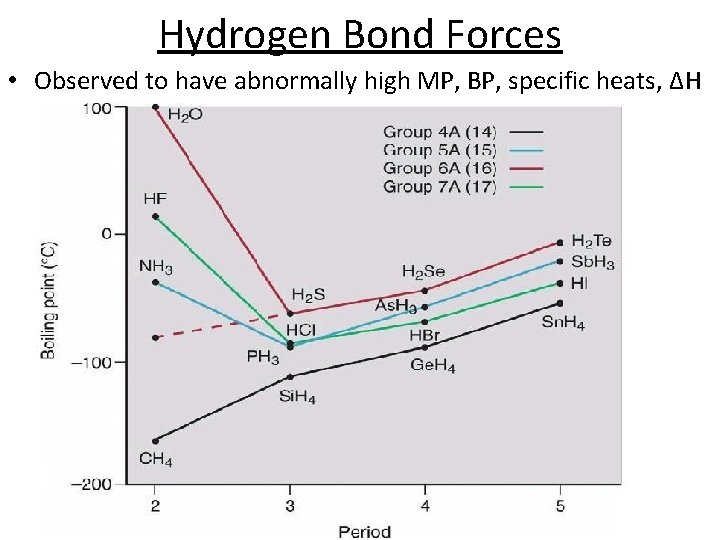
Hydrogen Bond Forces • Observed to have abnormally high MP, BP, specific heats, ΔH
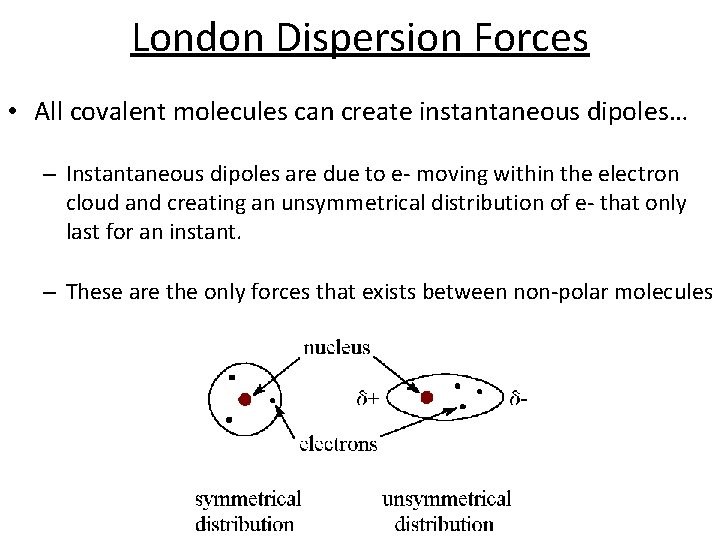
London Dispersion Forces • All covalent molecules can create instantaneous dipoles… – Instantaneous dipoles are due to e- moving within the electron cloud and creating an unsymmetrical distribution of e- that only last for an instant. – These are the only forces that exists between non-polar molecules
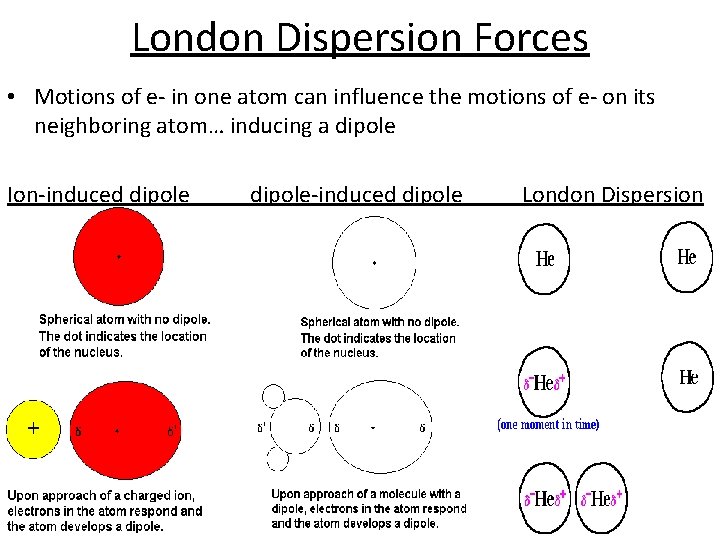
London Dispersion Forces • Motions of e- in one atom can influence the motions of e- on its neighboring atom… inducing a dipole Ion-induced dipole-induced dipole London Dispersion
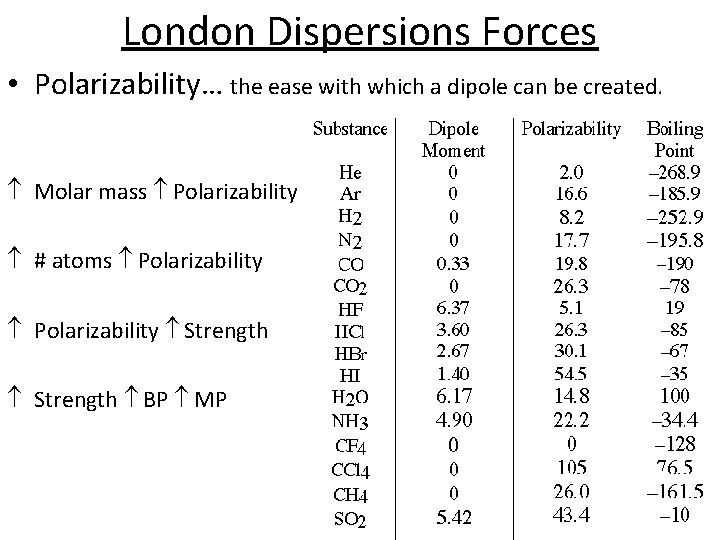
London Dispersions Forces • Polarizability… the ease with which a dipole can be created. Molar mass Polarizability # atoms Polarizability Strength BP MP
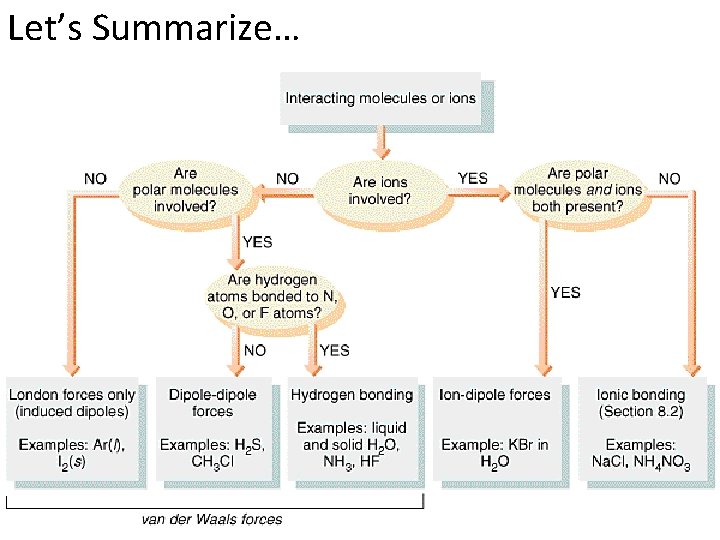
Let’s Summarize…
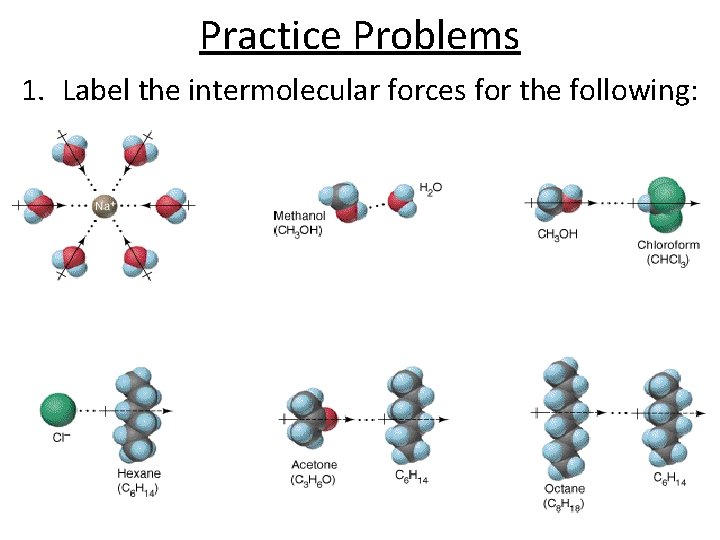
Practice Problems 1. Label the intermolecular forces for the following:

Practice Problems 2. The dipole moments of acetonitrile, CH 3 CN, and methyl iodide, CH 3 I are 3. 9 Debye and 1. 62 Debye, respectively. a) Which of these substances has the greater dipole-dipole attractions among its molecules? CH 3 CN larger dipole means stronger intermolecular attraction b) Which of these substances has greater London dispersion attractions? CH 3 I larger molar mass means stronger LDF c) The BP of CH 3 CN and CH 3 I are 354. 8 K and 315. 6 K, respectively. Which substance has the greater overall attractive forces? CH 3 CN dipole must have a greater affect than the LDF

Practice Problems 3. Of Br 2, Ne, HCl, HBr, and N 2, which is likely to have a. The largest intermolecular dispersion forces • Largest molar mass = Br 2 b. The largest dipole-dipole attractive forces • Largest Difference in electronegativity = HCl
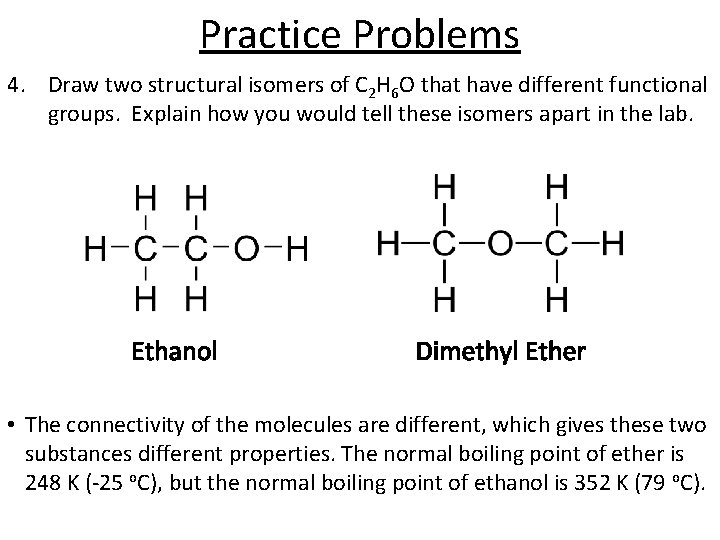
Practice Problems 4. Draw two structural isomers of C 2 H 6 O that have different functional groups. Explain how you would tell these isomers apart in the lab. Ethanol Dimethyl Ether • The connectivity of the molecules are different, which gives these two substances different properties. The normal boiling point of ether is 248 K (-25 o. C), but the normal boiling point of ethanol is 352 K (79 o. C).

Homework • Chapter 10 # 35 -40 all
Warm-Up 1. List the substances Ba. Cl 2, H 2, CO, HF, and Ne in order of increasing boiling points. H 2 < Ne < CO < HF < Ba. Cl 2 2. Of the following substances: CH 3, CH 3 OH, CH 3 CH 2 OH a. Identify the intermolecular attractions present LDF & H-bond b. Select the substance with the highest boiling CH 3 CH 2 OH – largest molar mass which means the LDF are the greatest and it has one H-bond
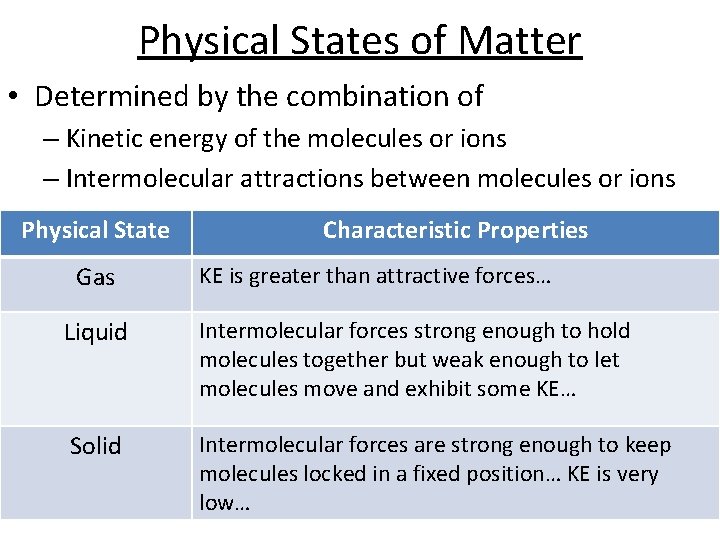
Physical States of Matter • Determined by the combination of – Kinetic energy of the molecules or ions – Intermolecular attractions between molecules or ions Physical State Gas Characteristic Properties KE is greater than attractive forces… Liquid Intermolecular forces strong enough to hold molecules together but weak enough to let molecules move and exhibit some KE… Solid Intermolecular forces are strong enough to keep molecules locked in a fixed position… KE is very low…

Liquids What we already know… • Intermolecular attractive forces hold molecules close together • Definite volume… indefinite shape • More dense but less compressible than gas • Able to be poured What we want to know… • Do all liquids flow the same? • Why does it hurt to belly flop in a pool? • Why do drops form? • Why do I always need to read the bottom of the meniscus? • Why do liquids evaporate even when it’s not hot enough to them boil? make
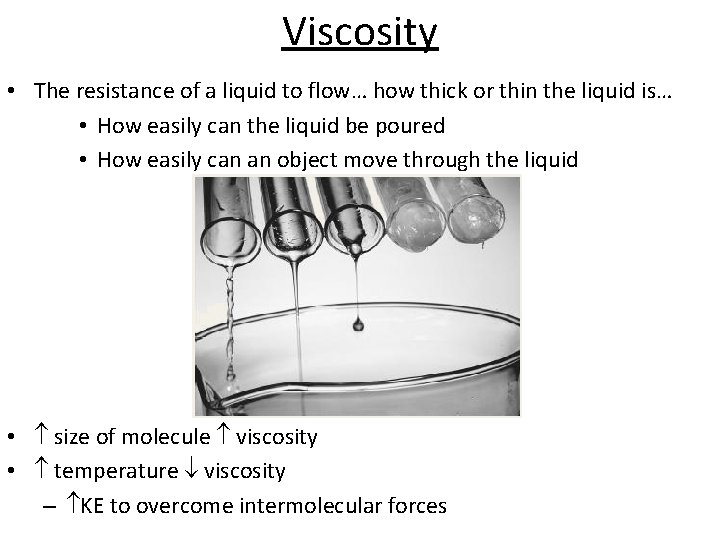
Viscosity • The resistance of a liquid to flow… how thick or thin the liquid is… • How easily can the liquid be poured • How easily can an object move through the liquid • size of molecule viscosity • temperature viscosity – KE to overcome intermolecular forces
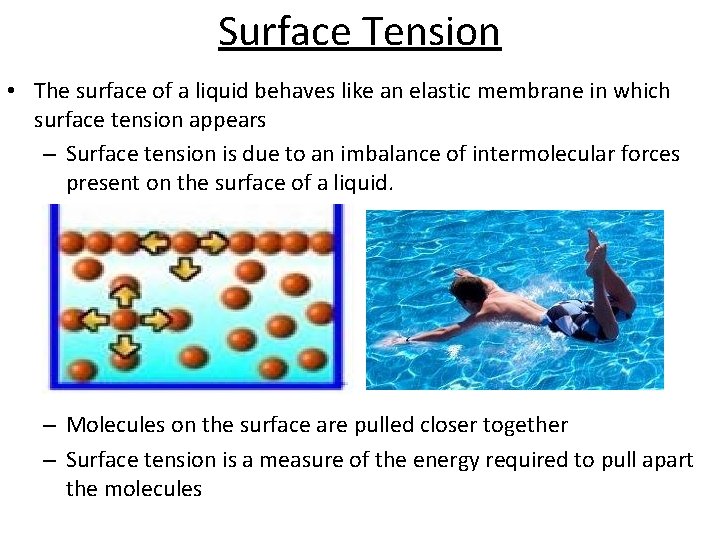
Surface Tension • The surface of a liquid behaves like an elastic membrane in which surface tension appears – Surface tension is due to an imbalance of intermolecular forces present on the surface of a liquid. – Molecules on the surface are pulled closer together – Surface tension is a measure of the energy required to pull apart the molecules
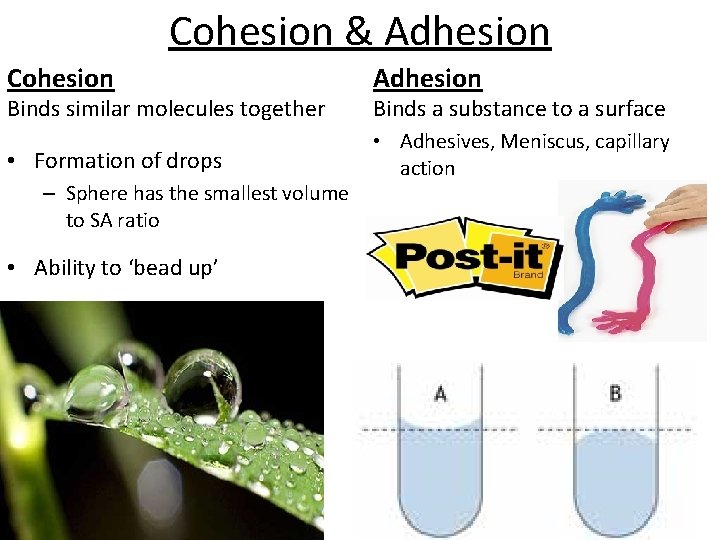
Cohesion & Adhesion Cohesion Adhesion • Formation of drops • Adhesives, Meniscus, capillary action Binds similar molecules together – Sphere has the smallest volume to SA ratio • Ability to ‘bead up’ Binds a substance to a surface
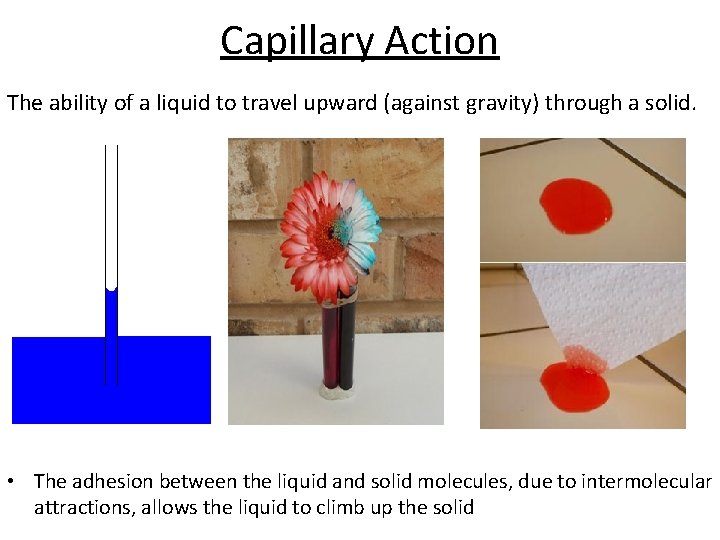
Capillary Action The ability of a liquid to travel upward (against gravity) through a solid. • The adhesion between the liquid and solid molecules, due to intermolecular attractions, allows the liquid to climb up the solid
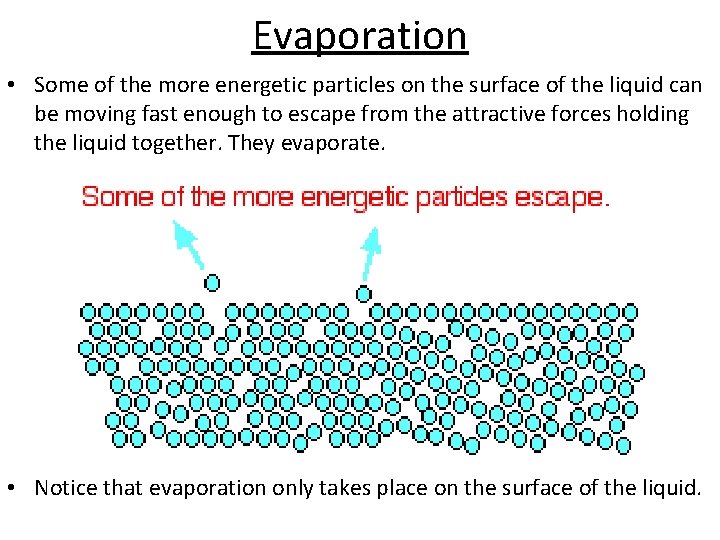
Evaporation • Some of the more energetic particles on the surface of the liquid can be moving fast enough to escape from the attractive forces holding the liquid together. They evaporate. • Notice that evaporation only takes place on the surface of the liquid.
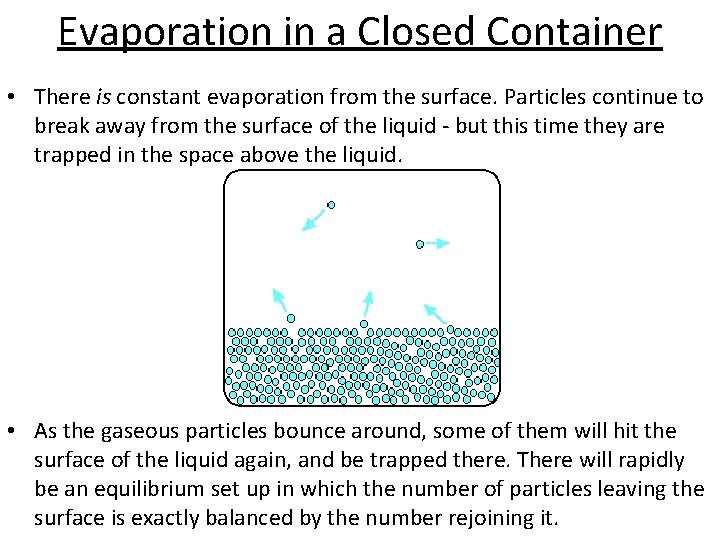
Evaporation in a Closed Container • There is constant evaporation from the surface. Particles continue to break away from the surface of the liquid - but this time they are trapped in the space above the liquid. • As the gaseous particles bounce around, some of them will hit the surface of the liquid again, and be trapped there. There will rapidly be an equilibrium set up in which the number of particles leaving the surface is exactly balanced by the number rejoining it.
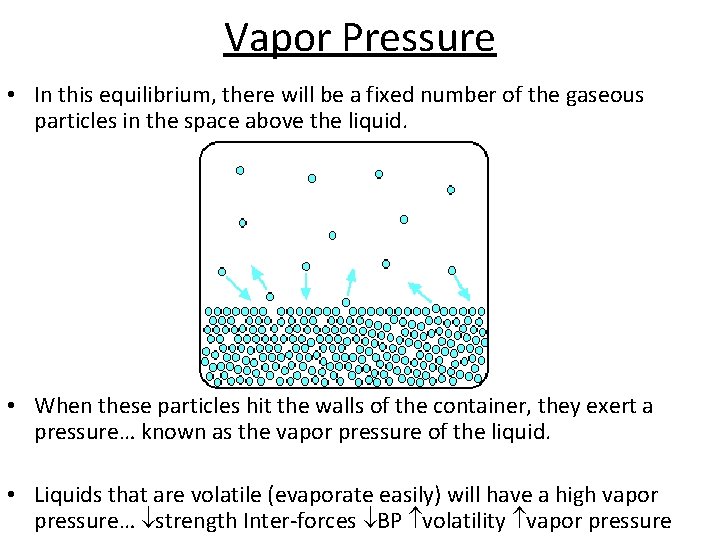
Vapor Pressure • In this equilibrium, there will be a fixed number of the gaseous particles in the space above the liquid. • When these particles hit the walls of the container, they exert a pressure… known as the vapor pressure of the liquid. • Liquids that are volatile (evaporate easily) will have a high vapor pressure… strength Inter-forces BP volatility vapor pressure

Practice Problems • Use the following liquids to answer the questions H 2 O CH 3 CH 2 OH CH 3 CH 2 CH 3 C 12 H 26 1. Which molecule is the most viscous? Dodecane… LDF & longest chain (easily tangled) 2. Which molecule is the most volatile? Propane… weakest intermolecular force & smaller (weaker) 3. Put the liquids in order from least to greatest vapor pressure? Water < ethanol < dodecane < propane 4. Which molecule has the most surface tension? Water… strongest intermolecular force
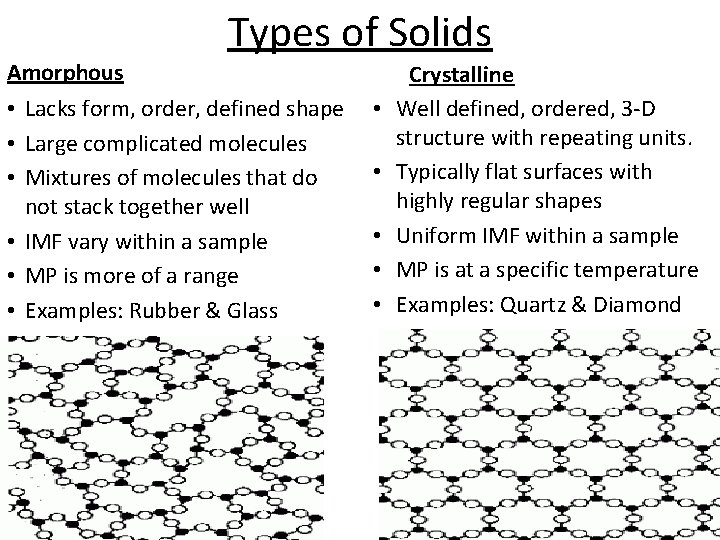
Amorphous Types of Solids • Lacks form, order, defined shape • Large complicated molecules • Mixtures of molecules that do not stack together well • IMF vary within a sample • MP is more of a range • Examples: Rubber & Glass • • • Crystalline Well defined, ordered, 3 -D structure with repeating units. Typically flat surfaces with highly regular shapes Uniform IMF within a sample MP is at a specific temperature Examples: Quartz & Diamond
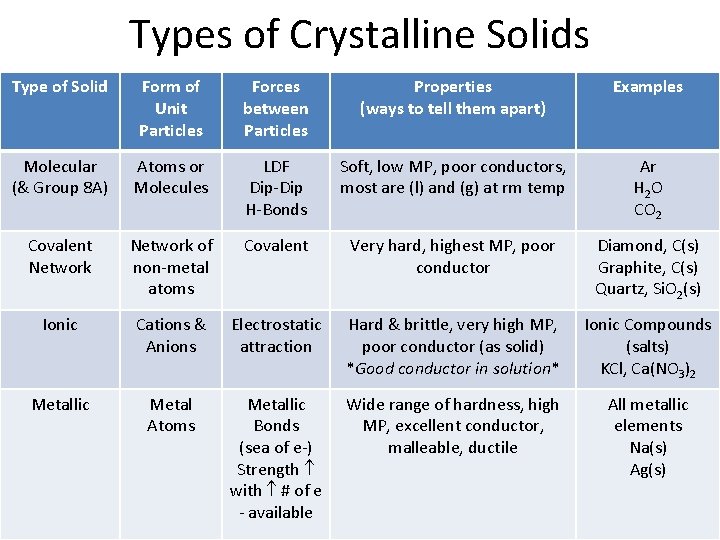
Types of Crystalline Solids Type of Solid Form of Unit Particles Forces between Particles Properties (ways to tell them apart) Examples Molecular (& Group 8 A) Atoms or Molecules LDF Dip-Dip H-Bonds Soft, low MP, poor conductors, most are (l) and (g) at rm temp Ar H 2 O CO 2 Covalent Network of non-metal atoms Covalent Very hard, highest MP, poor conductor Diamond, C(s) Graphite, C(s) Quartz, Si. O 2(s) Ionic Cations & Anions Electrostatic attraction Hard & brittle, very high MP, poor conductor (as solid) *Good conductor in solution* Ionic Compounds (salts) KCl, Ca(NO 3)2 Metallic Metal Atoms Metallic Bonds (sea of e-) Strength with # of e - available Wide range of hardness, high MP, excellent conductor, malleable, ductile All metallic elements Na(s) Ag(s)
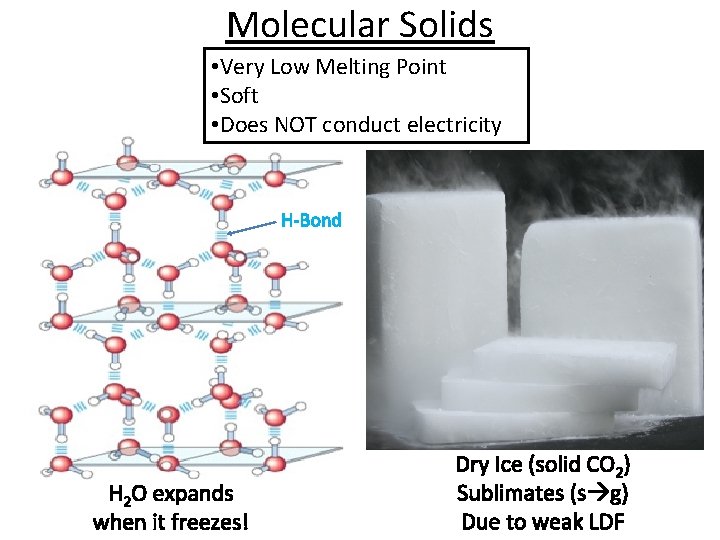
Molecular Solids • Very Low Melting Point • Soft • Does NOT conduct electricity H-Bond H 2 O expands when it freezes! Dry Ice (solid CO 2) Sublimates (s g) Due to weak LDF
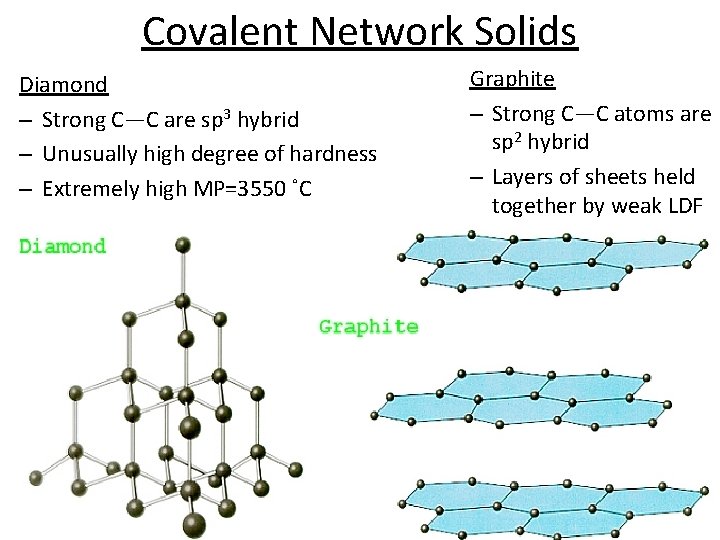
Covalent Network Solids Diamond – Strong C—C are sp 3 hybrid – Unusually high degree of hardness – Extremely high MP=3550 ˚C Graphite – Strong C—C atoms are sp 2 hybrid – Layers of sheets held together by weak LDF
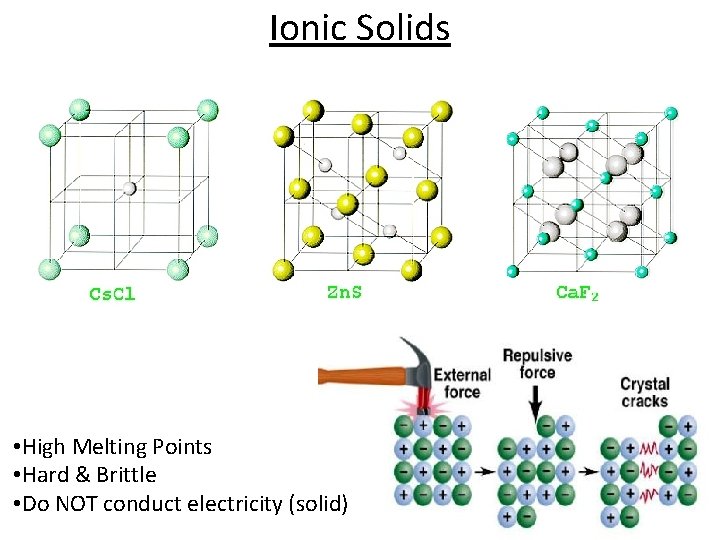
Ionic Solids • High Melting Points • Hard & Brittle • Do NOT conduct electricity (solid)
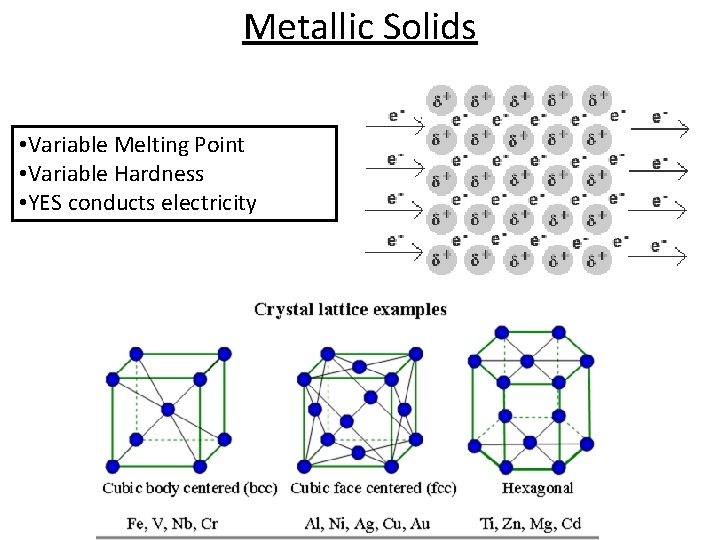
Metallic Solids • Variable Melting Point • Variable Hardness • YES conducts electricity
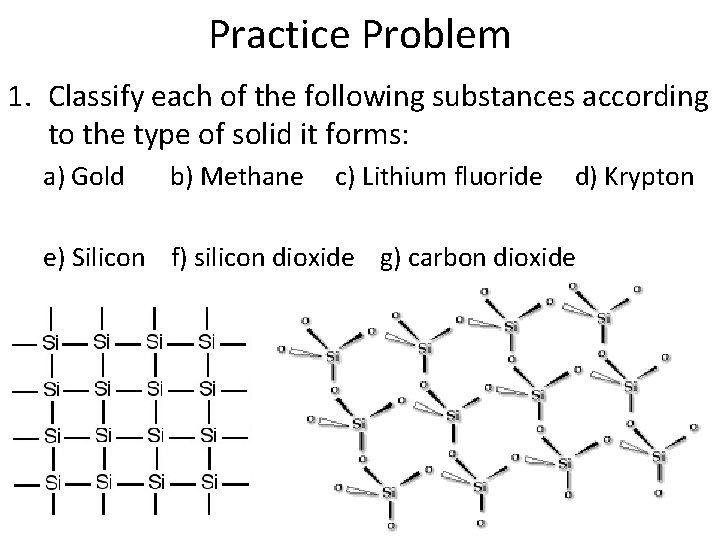
Practice Problem 1. Classify each of the following substances according to the type of solid it forms: a) Gold b) Methane c) Lithium fluoride d) Krypton e) Silicon f) silicon dioxide g) carbon dioxide

Homework • Outline Chapter 10 Sections 5 & 6 • Chapter 10 # 41 -44 all
- Slides: 40