Whats the difference between kinetic and potential energy
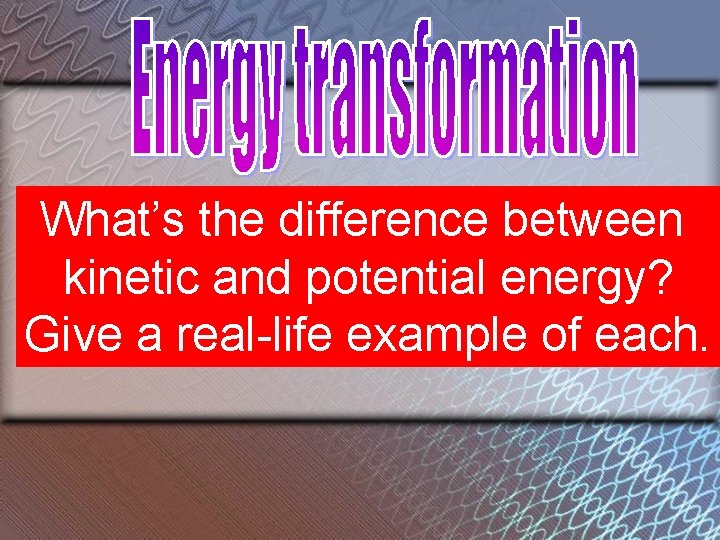
What’s the difference between kinetic and potential energy? Give a real-life example of each.

EXAMPLES SKIING DOWNHILL WATER FLOWING IN A RIVER EROSION

EXAMPLES AT THE TOP OF A SKI SLOPE WATER BEHIND A DAM DEPOSITION
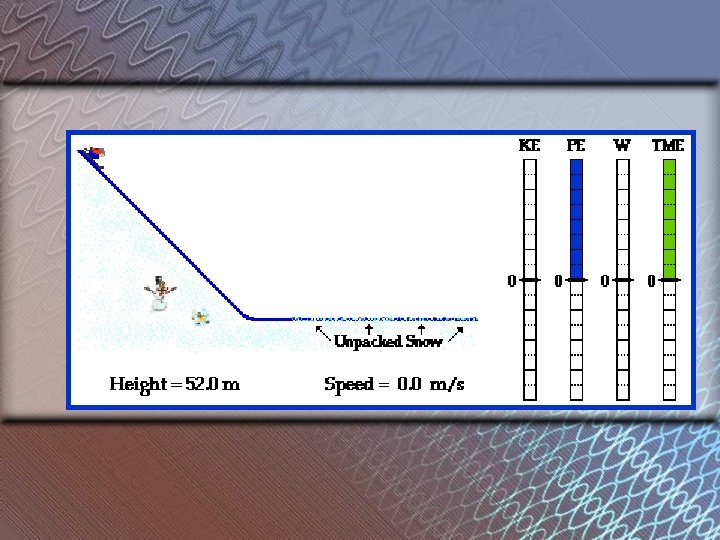
What is the relationship between Potential Energy and height? Veruckt!
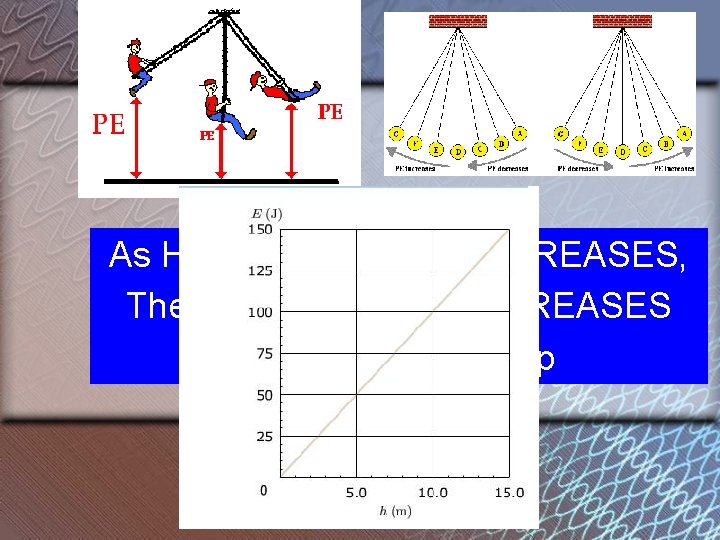
As Height of an object INCREASES, The potential energy INCREASES • Direct relationship
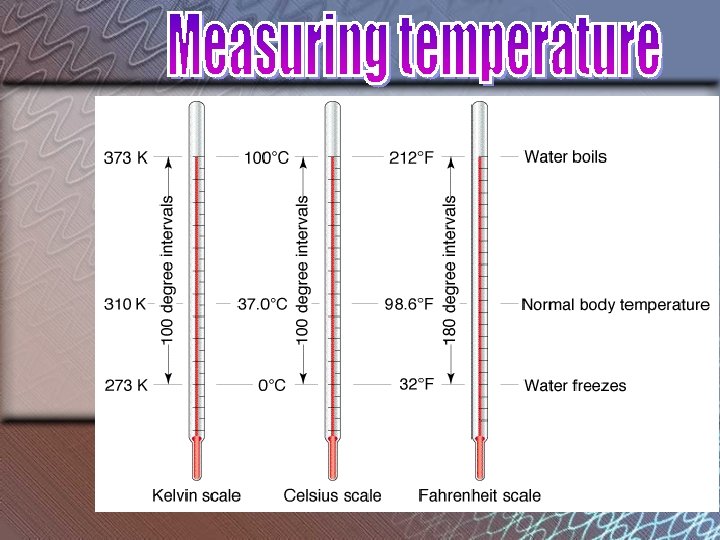
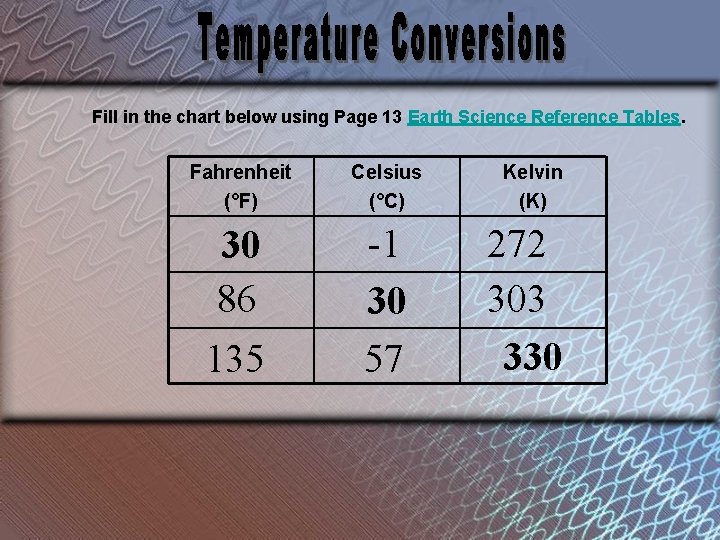
Fill in the chart below using Page 13 Earth Science Reference Tables. Fahrenheit (°F) Celsius (°C) 30 86 135 -1 30 57 Kelvin (K) 272 303 330
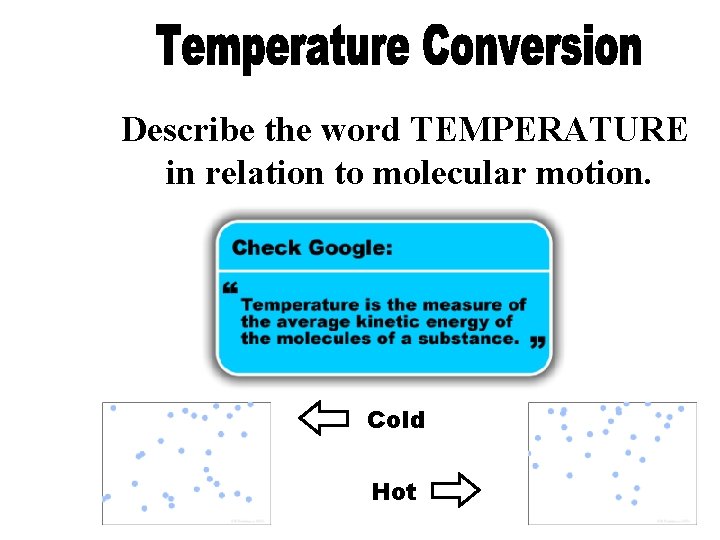
Describe the word TEMPERATURE in relation to molecular motion. Cold Hot
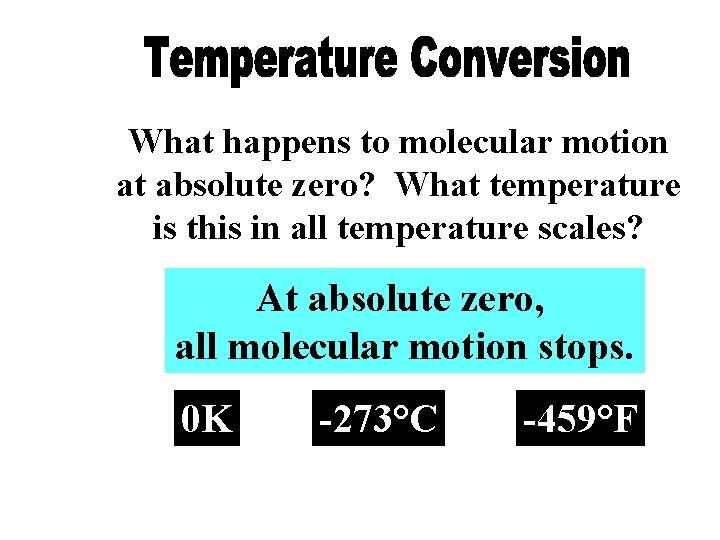
What happens to molecular motion at absolute zero? What temperature is this in all temperature scales? At absolute zero, all molecular motion stops. 0 K -273°C -459°F

Something to think about …. . ? ? ? Why do we here on Long Island not have many snow days? ? ?

the amount of energy required to raise the temperature of 1 gram of a substance 1°C Please go to Page 1 of the Earth Science Reference Tables.

Why does water heat up and cool down more slowly than land? Water has a higher specific heat

If you heated equal masses of basalt and lead, which one would record a faster increase in temperature? Explain how you know. the lead because it has a lower specific heat

Which Earth material has the greatest specific heat?

How does the high specific heat of water affect us here on Long Island every day? ? ? Let’s find out……. . Absorption and Radiation Lab

Where is the air warmer? cooler? Draw the convective cell

Where is the air warmer? Cooler?
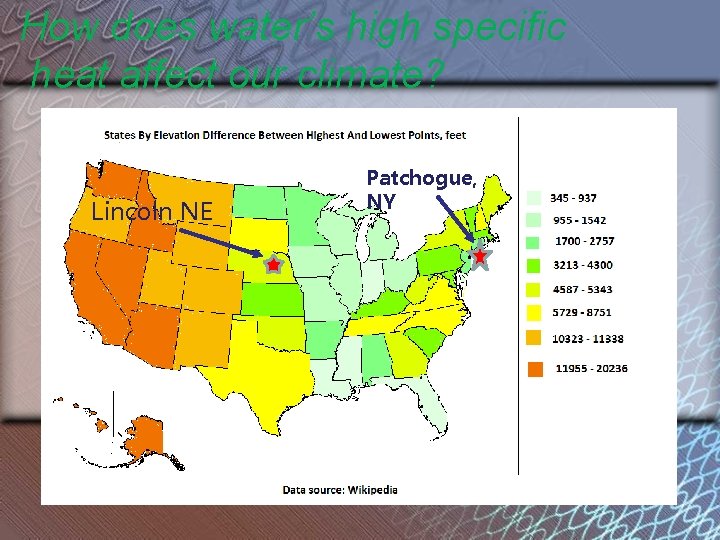
How does water’s high specific heat affect our climate? Lincoln NE Patchogue, NY
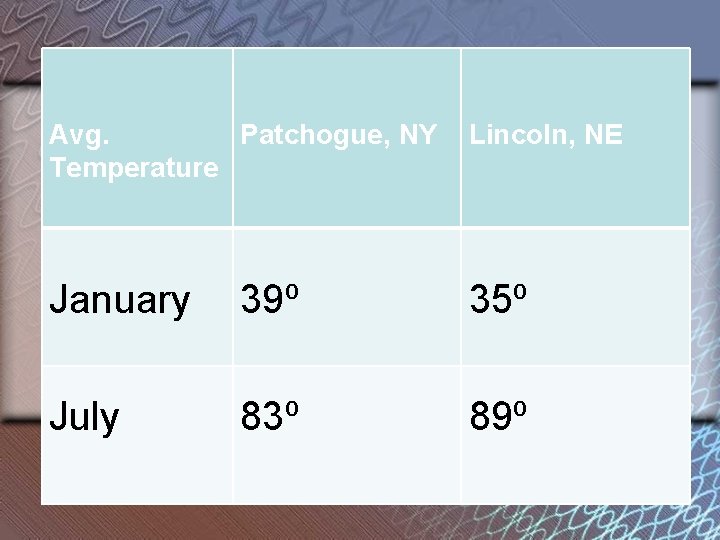
Avg. Patchogue, NY Temperature Lincoln, NE January 39⁰ 35⁰ July 83⁰ 89⁰

Long Island has cooler summers and warmer winters than Nebraska because of water’s ______! high specific heat

Something to think about …. . World climate data

Properties of Water

Something to think about …. .
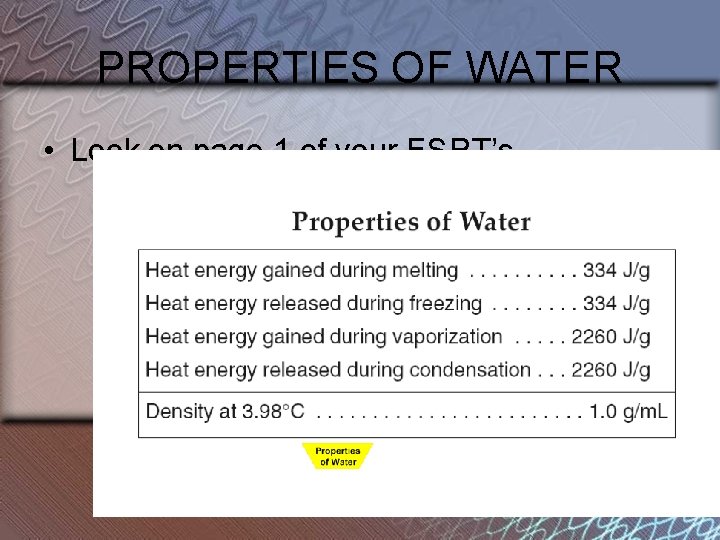
PROPERTIES OF WATER • Look on page 1 of your ESRT’s
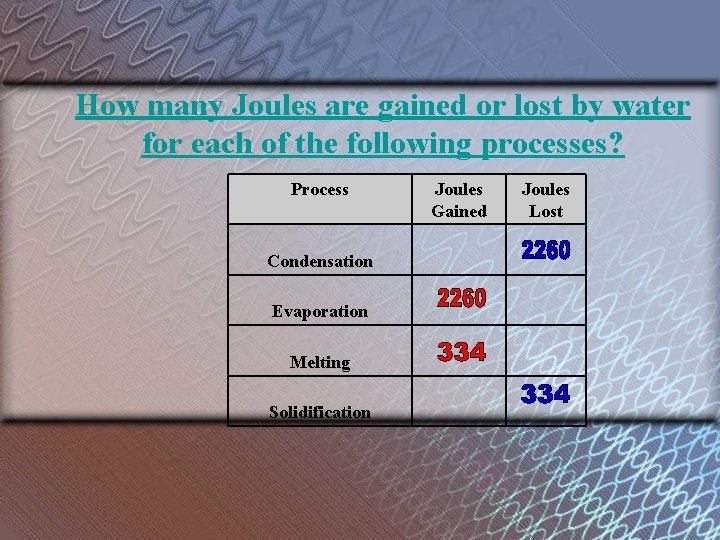
How many Joules are gained or lost by water for each of the following processes? Process Condensation Evaporation Melting Solidification Joules Gained Joules Lost
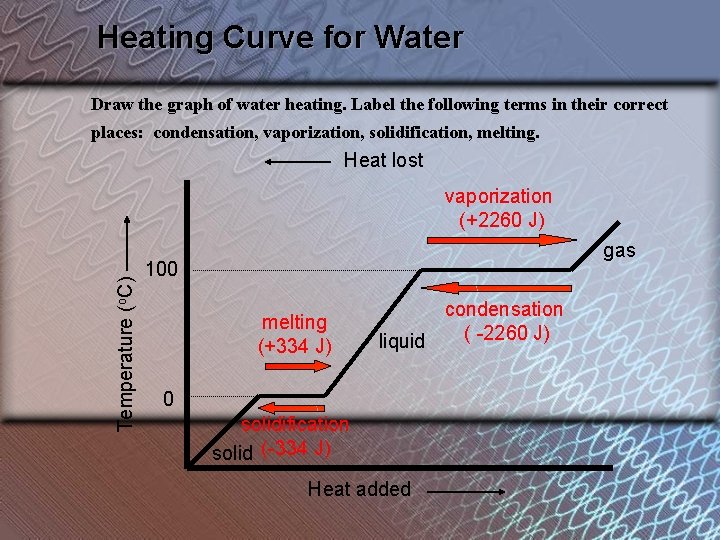
Heating Curve for Water Draw the graph of water heating. Label the following terms in their correct places: condensation, vaporization, solidification, melting. Heat lost Temperature (o. C) vaporization (+2260 J) gas 100 melting (+334 J) condensation ( -2260 J) liquid 0 solidification solid (-334 J) Heat added
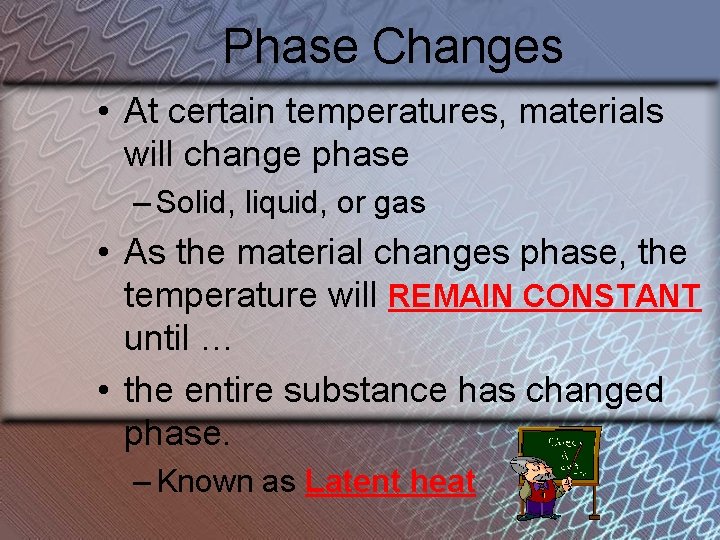
Phase Changes • At certain temperatures, materials will change phase – Solid, liquid, or gas • As the material changes phase, the temperature will REMAIN CONSTANT until … • the entire substance has changed phase. – Known as Latent heat

Something to think about …. . A burn from steam can be more severe than a burn from boiling water. Why? ? video
- Slides: 29