Whats the difference between kinetic and potential energy

What’s the difference between kinetic and potential energy? Give a real-life example of each.

EXAMPLES SKIING DOWNHILL WATER FLOWING IN A RIVER EROSION

EXAMPLES AT THE TOP OF A SKI SLOPE WATER BEHIND A DAM DEPOSITION
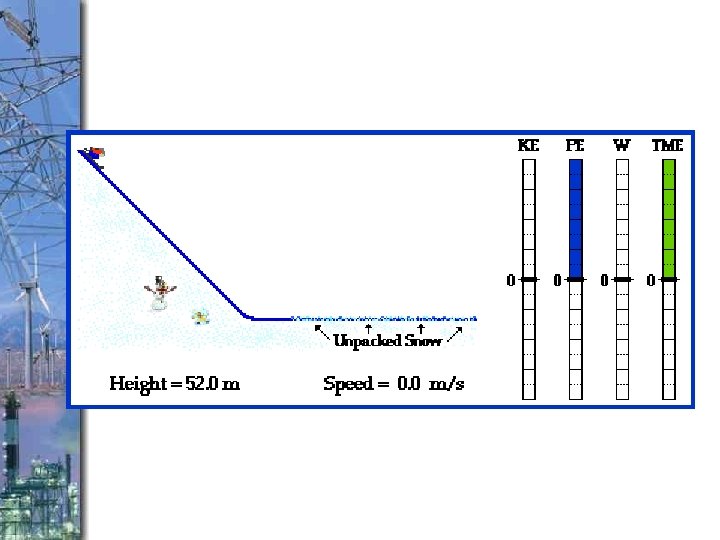
What is the relationship between Potential Energy and height? Veruckt!
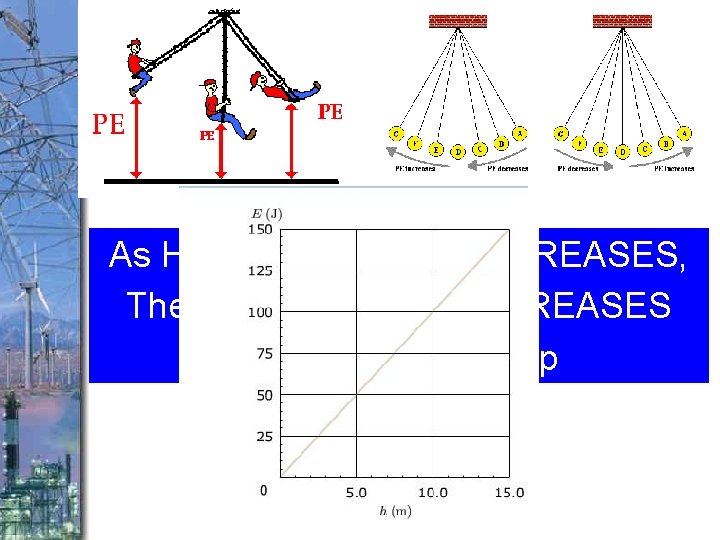
As Height of an object INCREASES, The potential energy INCREASES v. Direct relationship
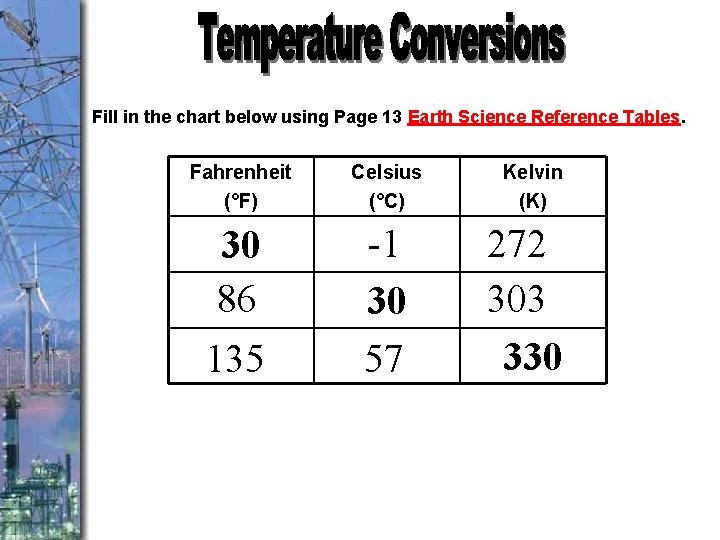
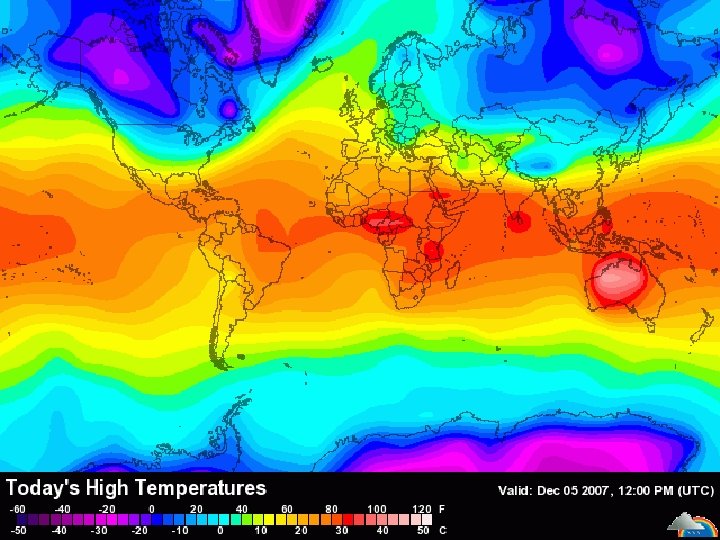
Fill in the chart below using Page 13 Earth Science Reference Tables. Fahrenheit (°F) Celsius (°C) 30 86 135 -1 30 57 Kelvin (K) 272 303 330
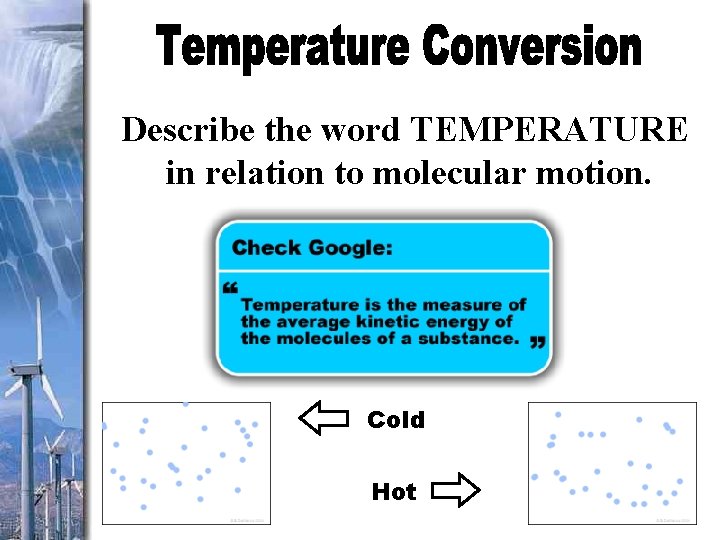
Describe the word TEMPERATURE in relation to molecular motion. Cold Hot
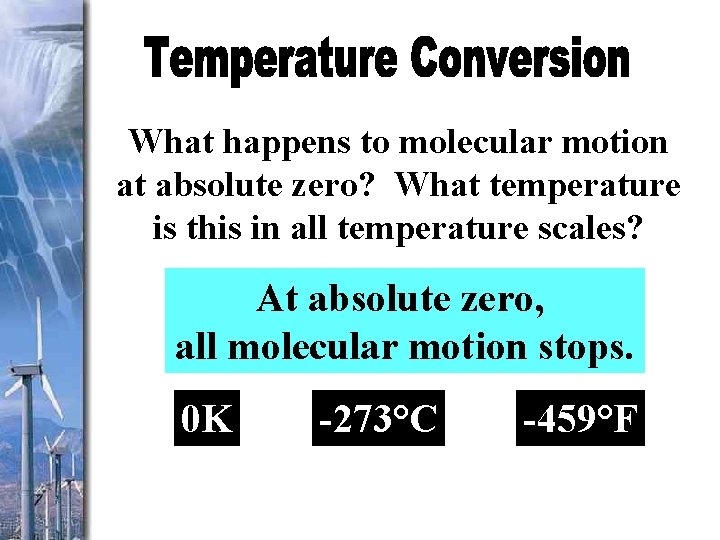
What happens to molecular motion at absolute zero? What temperature is this in all temperature scales? At absolute zero, all molecular motion stops. 0 K -273°C -459°F

the amount of energy required to raise the temperature of 1 gram of a substance 1°C Please go to Page 1 of the Earth Science Reference Tables.

Why does water heat up and cool down slower than land? Water has a higher specific heat

If you heated equal masses of basalt and lead, which one would record a faster increase in temperature? Explain how you know. the lead because it has a lower specific heat

Which Earth material has the greatest specific heat?
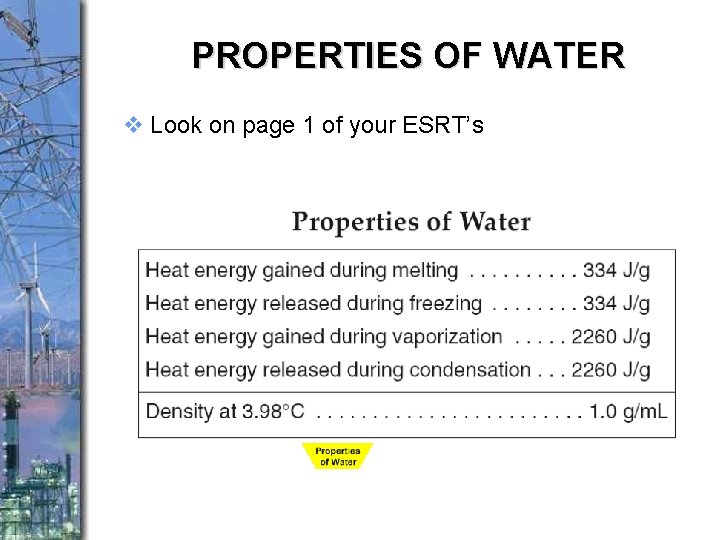
PROPERTIES OF WATER v Look on page 1 of your ESRT’s
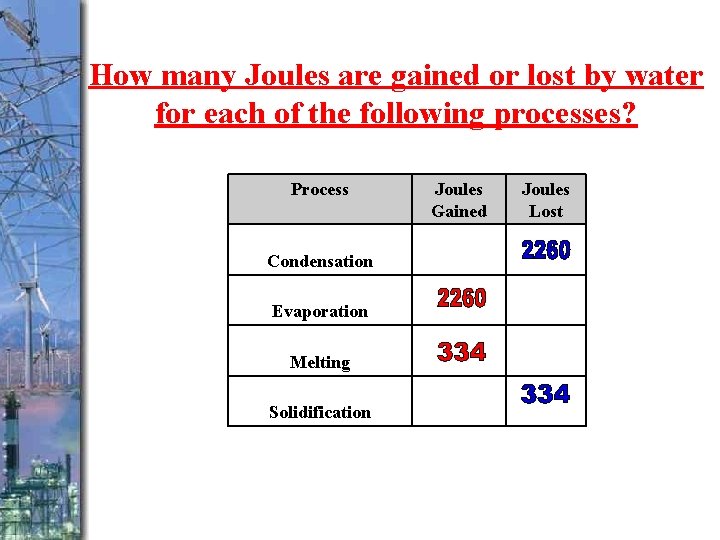
How many Joules are gained or lost by water for each of the following processes? Process Condensation Evaporation Melting Solidification Joules Gained Joules Lost
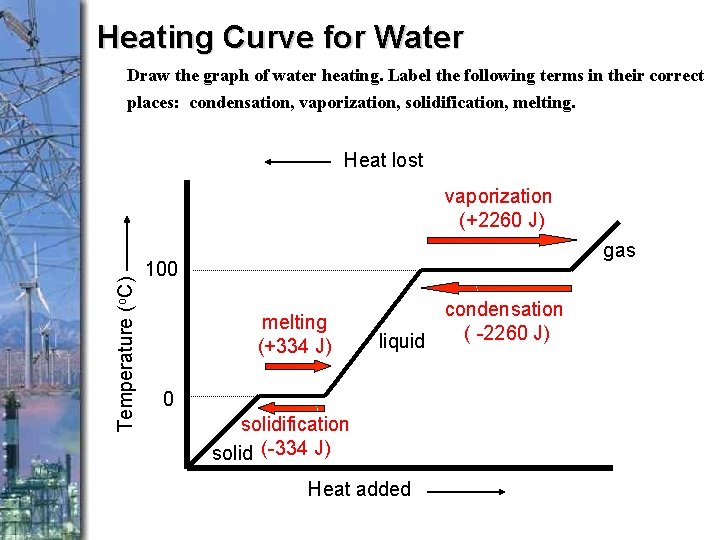
Heating Curve for Water Draw the graph of water heating. Label the following terms in their correct places: condensation, vaporization, solidification, melting. Heat lost Temperature (o. C) vaporization (+2260 J) gas 100 melting (+334 J) condensation ( -2260 J) liquid 0 solidification solid (-334 J) Heat added
Phase Changes v. At certain temperatures materials will change phase ● Solid, liquid, or gas v. As the material changes phase, the temperature will REMAIN CONSTANT until … vthe entire substance has changed phase. ● Known as Latent heat
How does heat move? ? v. Energy transfer ●Always moves from HIGH to LOW ENERGY ●Energy SOURCE to energy SINK ●HOT TO COLD

What are three ways that energy can be transferred between objects? Give one real-life example of each.

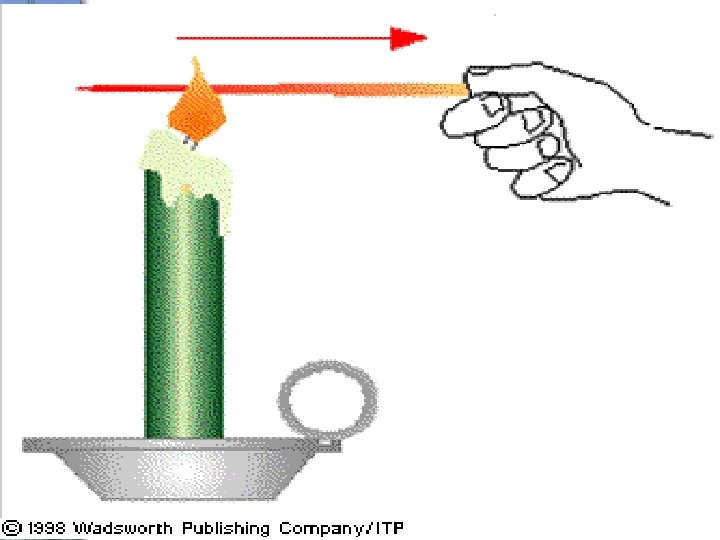
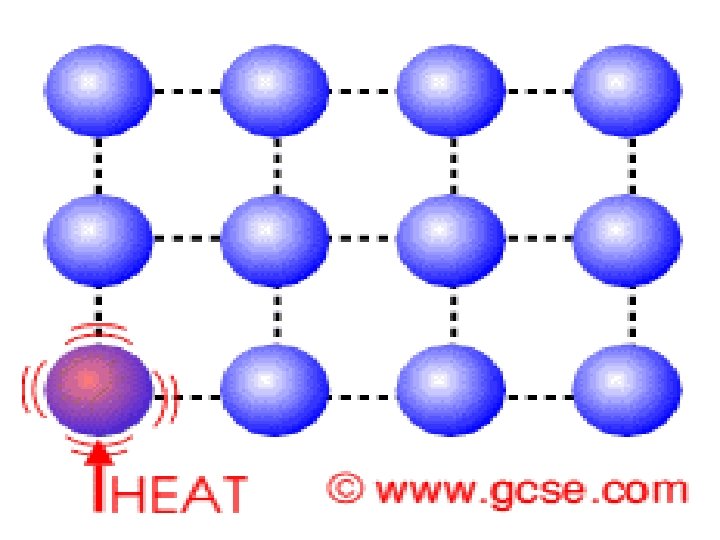
occurs in ________________of molecules Example:

occurs in ___________ caused by __________
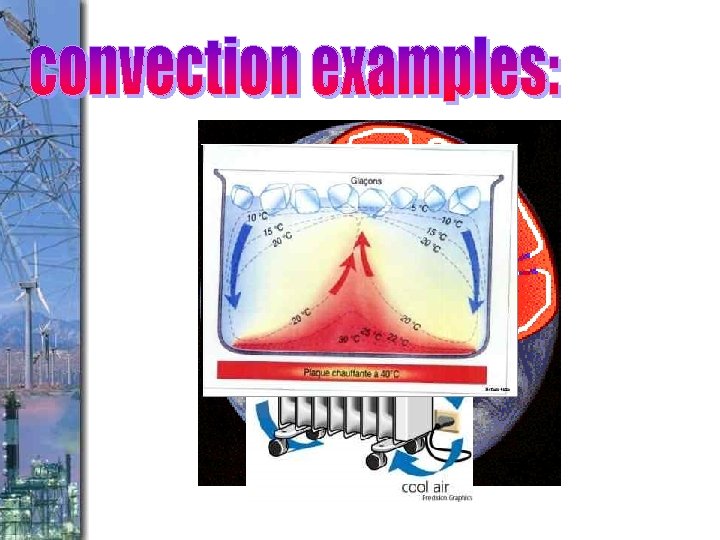

From where do convection ovens heat? From the top or the bottom? From where do refrigerators cool? From the top or the bottom?

travel by _______ No molecules are needed Examples:


No energy leaves the system Energy is lost to the environment

v. Energy Transfer Website v. Sing it out!
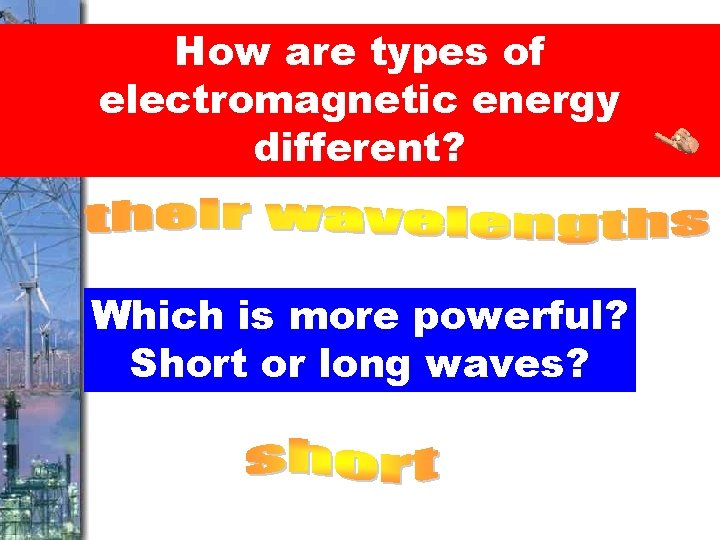
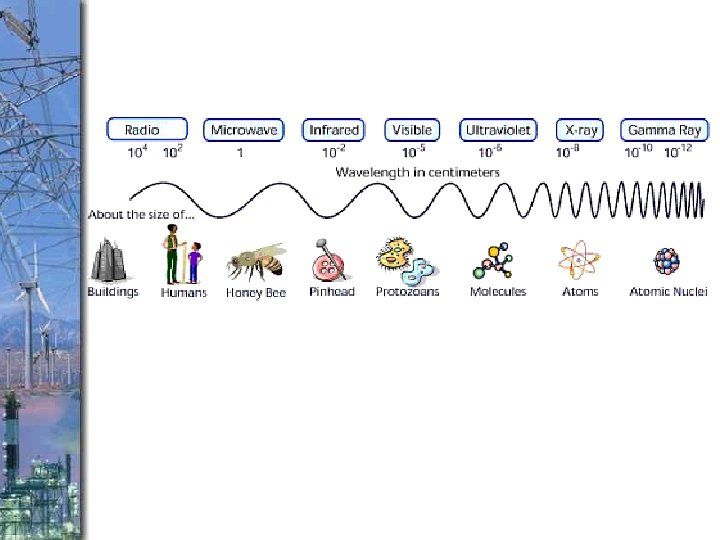
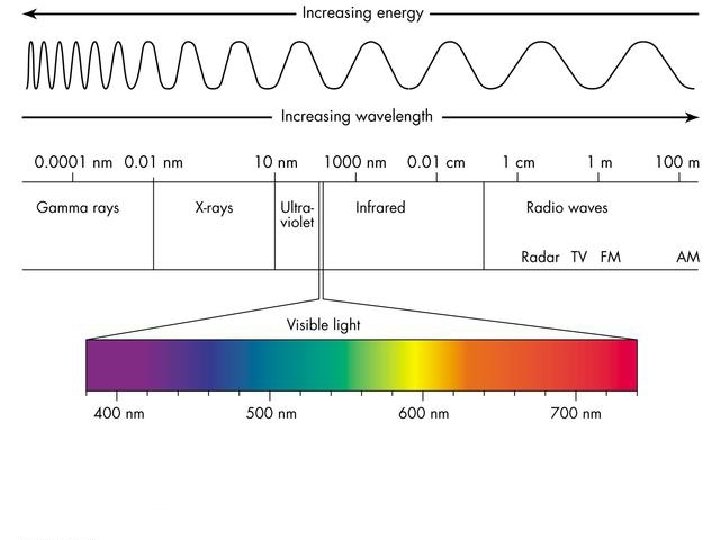
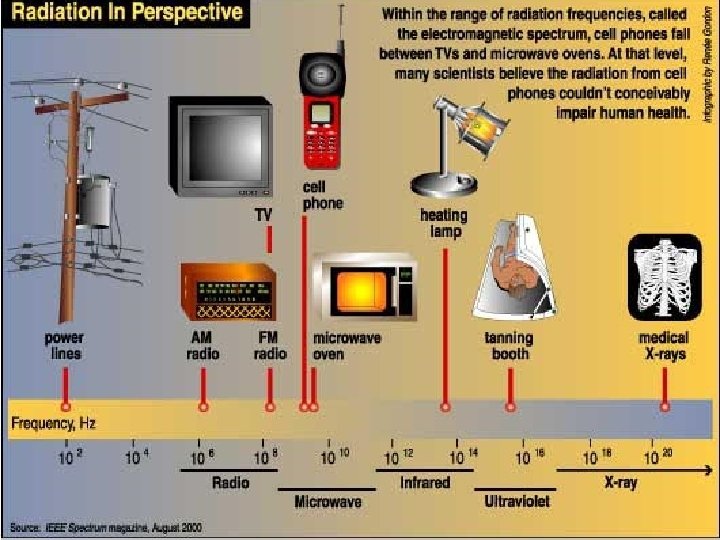
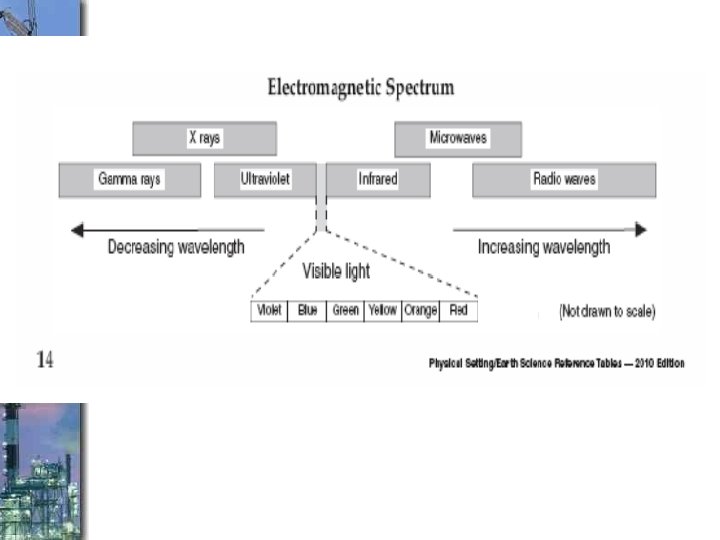
How are types of electromagnetic energy different? Which is more powerful? Short or long waves?

Where do we get our energy from? ? ? v. External heat- SUN ●Nuclear Fusion v. Internal Heat- Geothermal Energy (heat from inside earth) ●Nuclear Fission

How does energy get from the Sun to the Earth?

Electromagnetic Energy v. What types of INSOLATION do we get from the sun? ●Infrared-heat ●Visible Light- The only type of energy we can see (sun gives off mostly this type energy) ●Ultra violet (UV)- burns skin v. Circle the type of energy we get the most of

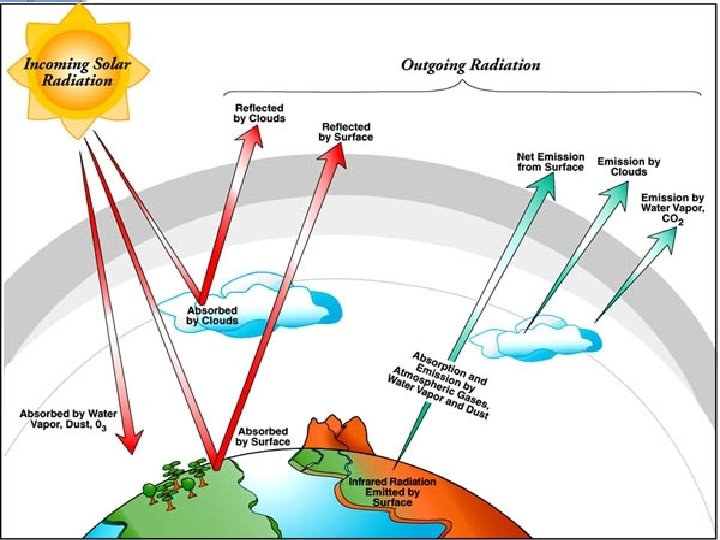
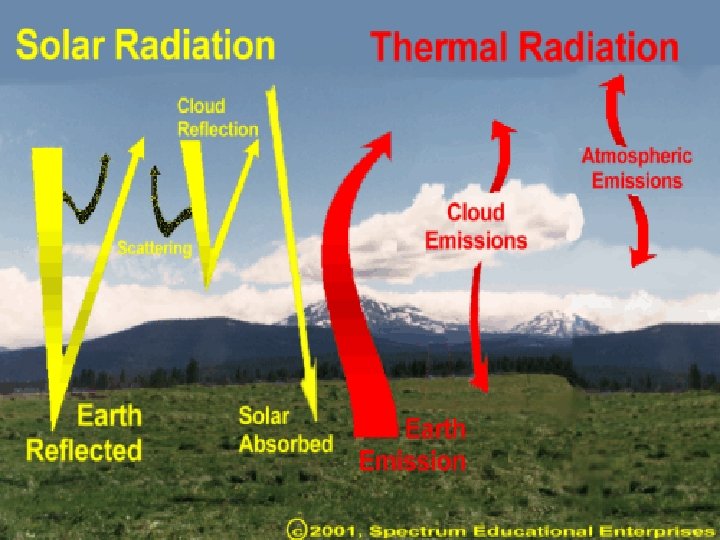
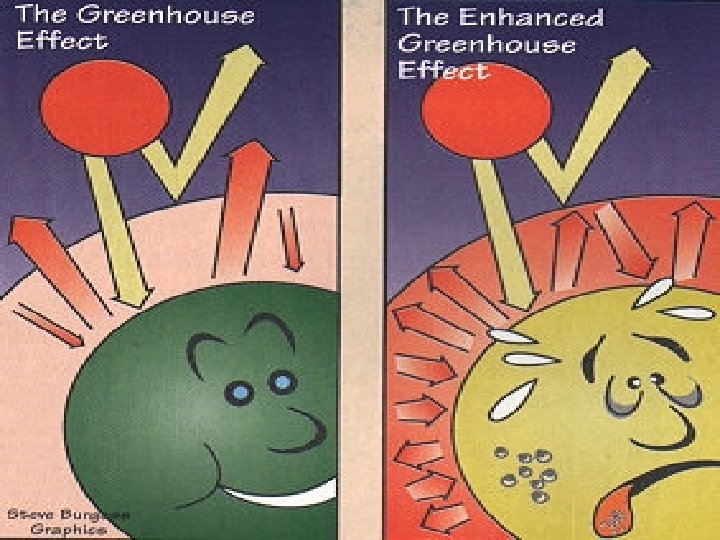
Most of sun’s energy will be… ●Absorbed § most UV rays are absorbed by the ozone layer § Water Vapor, Methane (CH 4), and Carbon Dioxide (CO 2) absorb infrared energy (greenhouse effect) ●Scattering§ pollution and aerosols also cause random scattering of insolation § That’s what makes those pretty sunsets!

v. Reflected- clouds “bounce” a lot of energy back to the atmosphere and even back to the surface v. Reflectedv. Light gets reflected by dust and aerosols in the atmosphere (pollution) v. Reflection on the surface of the earth!! ●Snow, oceans, light and smooth surfaces reflect insolation

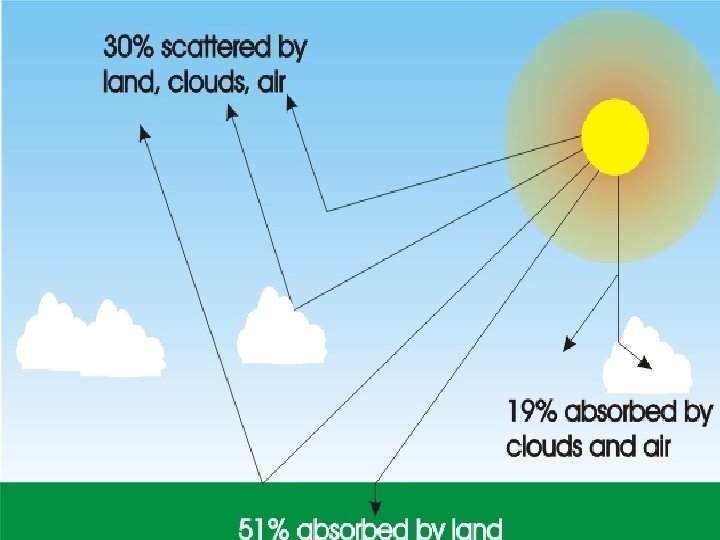
Insolation reaching Earth’s surface v. Only half of the Insolation coming into our atmosphere reaches the surface of Earth.
Absorbed vs. Reflected v. Surface characteristics of an object determine whether energy is absorbed or reflected v. What surface characteristics would absorb a lot of energy?

dark & rough light colored & smooth


v. SOMETHING THAT ABSORBS ENERGY QUICKLY, v. MUST GIVE IT BACK QUICKLY v. ANY type of energy that is absorbed becomes RE-radiated as LONGWAVE infrared (heat) energy.
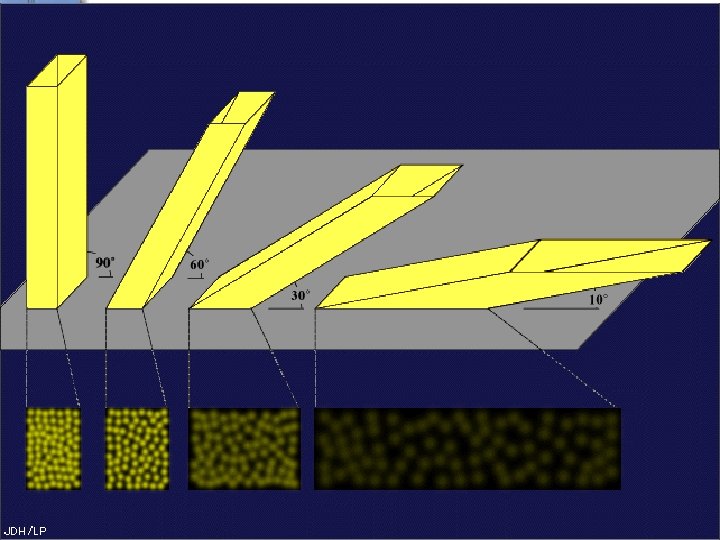
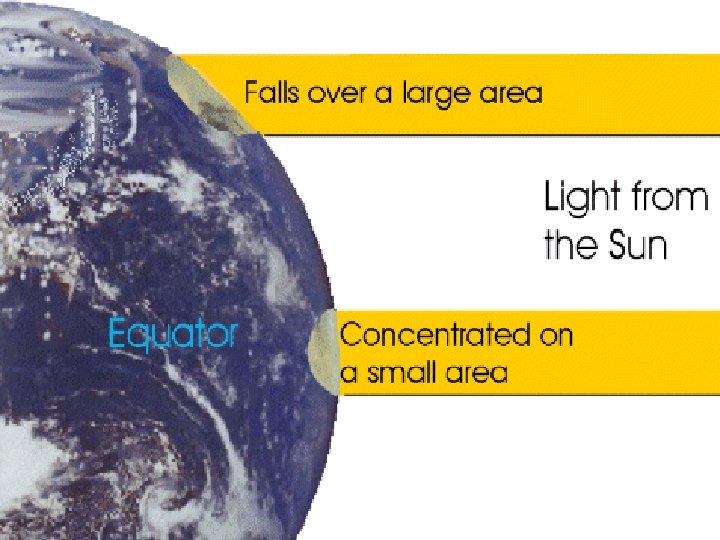
Angle of Incidence ●Altitude ●the height of the sun above the horizon ●The higher the sun, The more intense the insolation (more absorbtion) ●Sun is the most intense at § Solar noon § The equator § Summertime
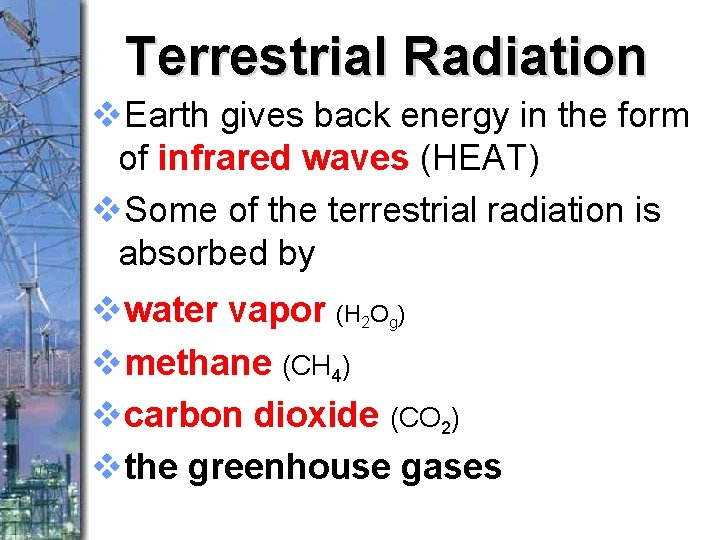
Terrestrial Radiation v. Earth gives back energy in the form of infrared waves (HEAT) v. Some of the terrestrial radiation is absorbed by vwater vapor (H 2 Og) vmethane (CH 4) vcarbon dioxide (CO 2) vthe greenhouse gases
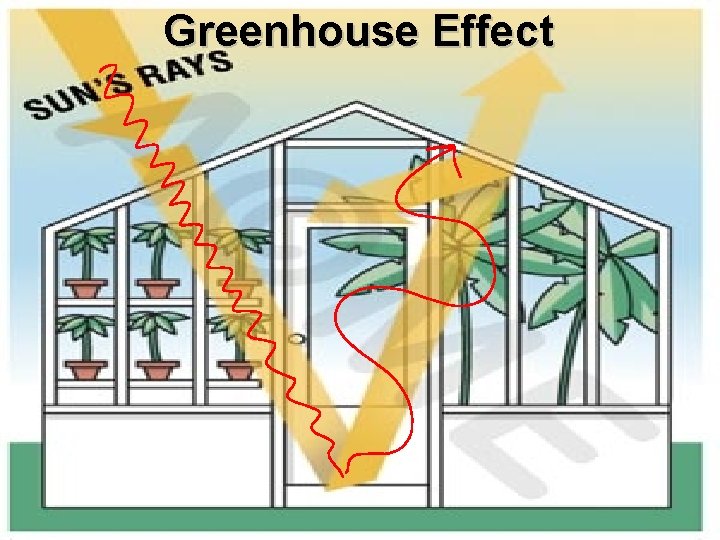
Greenhouse Effect
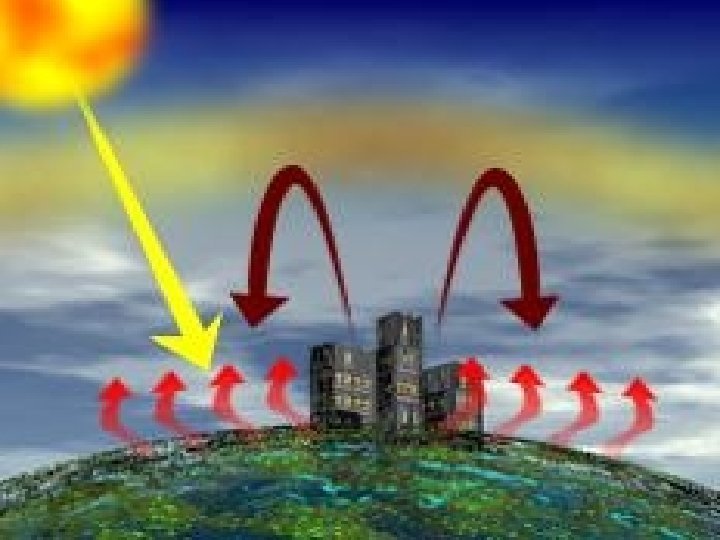

Greenhouse Effect v. Short wave UV Radiation comes in v. And longwave infrared radiation trapped by the greenhouse gases v. The greenhouse effect is a normal, needed process on earth v. The increase of the greenhouse gases like CH 4 and CO 2 causes GLOBAL WARMING!!! v. Global warming has nothing to do with the ozone layer
Insolation Factors Two main factors affecting amount of insolation at a location are: v Angle of Insolation: ● greater angle = more insolation. The higher the sun is in the sky, the greater level of insolation. v Duration of Insolation: v more hours of sunlight = greater insolation ● We have more hours of daylight in the summer when it is warmer

23. 5°N-23. 5°S- Tropics 66. 5°N & S-90°- Polar In between is known as the temperate zones (has 4 seasons)
- Slides: 61