Whats new in asthma Wendy Pigg Practice support








- Slides: 15

What’s new in asthma Wendy Pigg Practice support Pharmacist/Independent Prescriber

NICE Quality standards Qs 25 � Launched in February 2013 � Provides best clinical practice statements for a health topic Statement 1. People with newly diagnosed asthma are diagnosed in accordance with BTS/SIGN guidance. Statement 2. Adults with new onset asthma are assessed for occupational causes. Statement 3. People with asthma receive a written personalised action plan. Statement 4. People with asthma are given specific training and assessment in inhaler technique before starting any new inhaler treatment. Statement 5. People with asthma receive a structured review at least annually. Statement 6. People with asthma who present with respiratory symptoms receive an assessment of their asthma control. Statement 7. People with asthma who present with an exacerbation of their symptoms receive an objective measurement of severity at the time of presentation. Statement 8. People aged 5 years or older presenting to a healthcare professional with a severe or life-threatening acute exacerbation of asthma receive oral or intravenous steroids within 1 hour of presentation. Statement 9. People admitted to hospital with an acute exacerbation of asthma have a structured review by a member of a specialist respiratory team before discharge. Statement 10. People who received treatment in hospital or through out-of-hours services for an acute exacerbation of asthma are followed up by their own GP practice within 2 working days of treatment. Statement 11. People with difficult asthma are offered an assessment by a multidisciplinary difficult asthma service. Ref : http: //guidance. nice. org. uk/QS 25

New licensing for Fostair® �The product license for the p. MDI Fostair® (beclometasone and formoterol) dose inhaler (p. MDI) has been updated with a new indication to allow use as maintenance and reliever therapy. �Dose is 1 inhalation of the inhaler regularly twice daily for maintenance and then take up to 6 additional inhalations as needed in response to symptoms. �Symbicort® DPI are already licensed in this way (SMART). The license extension for Fostair® now provides a patients choice of delivery device (p. MDI or DPI). �Patients must be carefully selected and patient education is key to the success of the regime which in the studies can provide an overall lower steroid load to patients.

New Inhalers - Flutiform®

New Inhalers - Flutiform® � Combination MDI inhaler containing fluticasone and formoterol �Available in 3 strengths, with a dose schedule of two puffs twice daily. (120 dose) �Flutiform 50 mcg/5 mcg and 125 mcg/5 mcg are licensed for use in adults and adolescents aged > 12 years. �Flutiform 250 microgram/10 microgram is licensed for use only in adults.

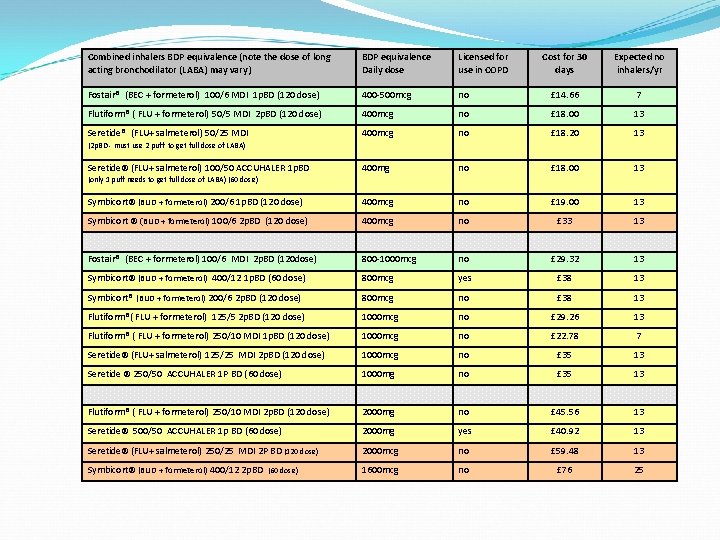
Combined inhalers BDP equivalence (note the dose of long acting bronchodilator (LABA) may vary) BDP equivalence Daily dose Licensed for use in COPD Cost for 30 days Expected no inhalers/yr Fostair® (BEC + formeterol) 100/6 MDI 1 p. BD (120 dose) 400 -500 mcg no £ 14. 66 7 Flutiform® ( FLU + formeterol) 50/5 MDI 2 p. BD (120 dose) 400 mcg no £ 18. 00 13 Seretide® (FLU+ salmeterol) 50/25 MDI 400 mcg no £ 18. 20 13 400 mg no £ 18. 00 13 Symbicort® (BUD + formeterol) 200/6 1 p. BD (120 dose) 400 mcg no £ 19. 00 13 Symbicort ® (BUD + formeterol) 100/6 2 p. BD (120 dose) 400 mcg no £ 33 13 Fostair® (BEC + formeterol) 100/6 MDI 2 p. BD (120 dose) 800 -1000 mcg no £ 29. 32 13 Symbicort® (BUD + formeterol) 400/12 1 p. BD (60 dose) 800 mcg yes £ 38 13 Symbicort® (BUD + formeterol) 200/6 2 p. BD (120 dose) 800 mcg no £ 38 13 Flutiform®( FLU + formeterol) 125/5 2 p. BD (120 dose) 1000 mcg no £ 29. 26 13 Flutiform® ( FLU + formeterol) 250/10 MDI 1 p. BD (120 dose) 1000 mcg no £ 22. 78 7 Seretide® (FLU+ salmeterol) 125/25 MDI 2 p. BD (120 dose) 1000 mcg no £ 35 13 Seretide ® 250/50 ACCUHALER 1 P BD (60 dose) 1000 mg no £ 35 13 Flutiform® ( FLU + formeterol) 250/10 MDI 2 p. BD (120 dose) 2000 mg no £ 45. 56 13 Seretide® 500/50 ACCUHALER 1 p BD (60 dose) 2000 mg yes £ 40. 92 13 Seretide® (FLU+ salmeterol) 250/25 MDI 2 P BD (120 dose) 2000 mcg no £ 59. 48 13 Symbicort® (BUD + formeterol) 400/12 2 p. BD (60 dose) 1600 mcg no £ 76 25 (2 p. BD - must use 2 puff to get full dose of LABA) Seretide® (FLU+ salmeterol) 100/50 ACCUHALER 1 p. BD (only 1 puff needs to get full dose of LABA) (60 dose)

Worcestershire Spend on ICS- Cost effective prescribing �N. I. C. E states to use the combination inhaler that is least costly that is suitable for that patient. �All new starters for p. MDI ICS/LABA inhalers should be on Fostair®/Flutiform® �Switching can be an option in suitable patients now we have a fluticasone p. MDI alternative to Seretide® �Switching 50% of patients from Seretide® 250 evohaler to Flutiform® 250 could save £ 183, 249 �Switching 50% of patients from Seretide® 125 evohaler to Flutiform® 125 could save £ 62, 313

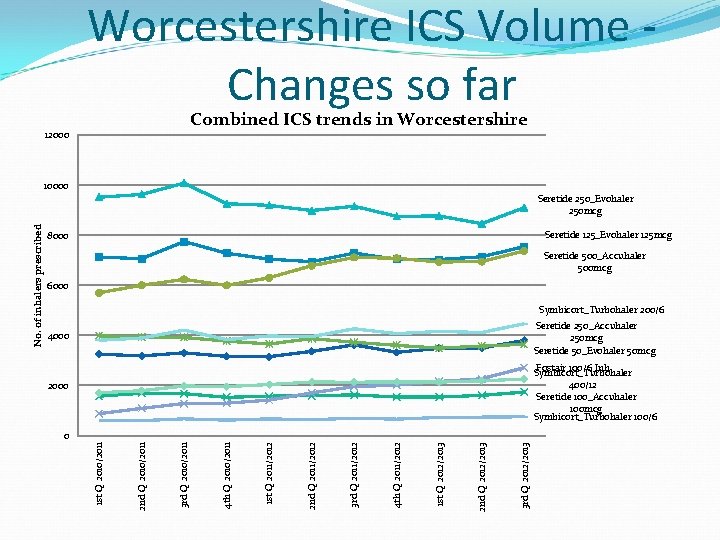
Worcestershire Spend on ICS- YTD Dec 2012 � The volumes of combined ICS is increasing year on year � 59% (49% in 2010) of total inhaled corticosteroid inhalers (ICS) prescribing is for combination inhalers. (Step 3 or more of BTS guidelines for asthma or COPD FEV 1 <50%) � This equates to a spend of £ 4, 490, 521 � £ 2. 2 million is spent on Seretide® 250 Evohaler/Seretide® 500 Accuhalers high dose inhaler ? ? Why ? ? � More patients moving from step 2 –to step 3 of BTS guidelines too early ? � Patients started on step 3 of BTS guidelines with higher dose steroid than needed ? � Patient started on higher dose steroids to gain control but not stepped down when stable � More COPD patients on triple therapy LAMA + ICS/LABA ? - limited evidence base for use

Q. I. P. P –High dose Inhaled corticosteroids in Asthma �QIPP – Quality, Innovation, Productivity and Prevention High dose = high cost = increased risk of side effects Numbers of patients with serious side effects may be small but Cost of fracture to the NHS Lifetime cost of a diabetic Cataract surgery cost

Worcestershire ICS Volume - Changes so far Combined ICS trends in Worcestershire 12000 10000 Seretide 125_Evohaler 125 mcg 8000 Seretide 500_Accuhaler 500 mcg 6000 Symbicort_Turbohaler 200/6 Seretide 250_Accuhaler 250 mcg Seretide 50_Evohaler 50 mcg 4000 Fostair 100/6 Inh Symbicort_Turbohaler 400/12 Seretide 100_Accuhaler 100 mcg Symbicort_Turbohaler 100/6 2000 3 rd Q 2012/2013 2 nd Q 2012/2013 1 st Q 2012/2013 4 th Q 2011/2012 3 rd Q 2011/2012 2 nd Q 2011/2012 1 st Q 2011/2012 4 th Q 2010/2011 3 rd Q 2010/2011 2 nd Q 2010/2011 0 1 st Q 2010/2011 No. of inhalers prescribed Seretide 250_Evohaler 250 mcg

BTS guidelines – Optimising Step 2 of treatment ? �Are we ensuring step 2 of BTS guidelines are followed with optimisation of ICS first before adding LABA. �Impact Project in Bristol found adequate inhaler training and education for these patients meant they gained control without need to step up. �In asthma reviews look at prescription ordering history – are patients actually using their ICS ? �Is stepping down ICS dose discussed and documented at every asthma review ? (is it on your templates ? ) �Is inhaler technique actually checked every time ?

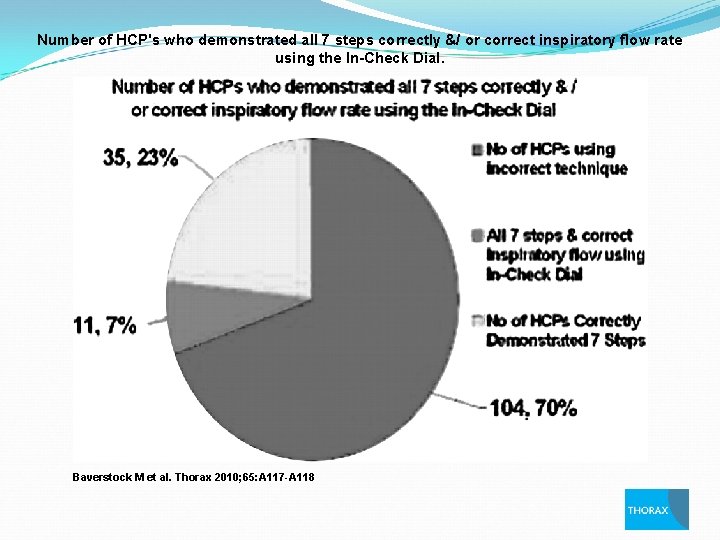
Number of HCP's who demonstrated all 7 steps correctly &/ or correct inspiratory flow rate using the In-Check Dial. Baverstock M et al. Thorax 2010; 65: A 117 -A 118

BTS guidelines – Step 3 of treatment �Ensure patient using at least 400 mcg BDP equivalent before adding LABA �No need to increase steroid when LABA added – therefore should be using low dose combined ICS inhalers which are more cost effective and lower doses of steroids. Fostair®/Flutiform 50®/Sertide 50®/Symbicort® �Step down when asthma control is achieved to reduce long term side effects of steroids.

BTS guidelines – Stepping up and stepping down �Stepping down 25% of patients from �Seretide® 250 Evohaler Seretide® 125 Evohaler = £ 161, 133 �Seretide® 250 Evohaler Flutiform® 125 Evohaler = £ 198, 908 �Seretide® 125 Evohaler Seretide®/Flutiform® 50 Evohaler = £ 92, 276 �Seretide® 250 Accuhaler Seretide® 100 accuhaler = £ 91, 000

Key messages �Asthma is a variable disease so patients can be over treated or undertreated. �In stable asthma with no symptoms consider step down in treatment every three months , decreasing dose by 25 -50% at a time. �Scottish study in 259 stable asthma patients on high dose ICS (>1200 BDP) had 50% reduction in dose and in 1 year no difference in exacerbation rate, GP attendance or health status SGRQ �If inhaler technique not correct – treatment is in vain. Hawkins et al BMJ 2003: 325: 1115 15