Whats New and Whats Next in the Treatment


















- Slides: 58

What’s New and What’s Next in the Treatment of MDS? Mikkael A. Sekeres, MD, MS Professor of Medicine Director, Leukemia Program Vice Chair for Clinical Research Cleveland Clinic Cleveland, Ohio David P. Steensma, MD, FACP Associate Professor of Medicine Harvard Medical School Institute Physician Dana-Farber Cancer Institute Boston, Massachusetts

Use of these Slides • Learners are welcome to use and share these slides in full or in part for educational purposes in noncommercial discussions with colleagues or patients. • The materials presented may discuss uses and dosages for therapeutic products that have not been approved by the United States Food and Drug Administration. Readers should verify all information and data before treating patients or using any therapies described in these materials. • The materials published reflect the views of the authors and not those of Medi. Com Worldwide, Inc. or the companies providing educational grant support. • These slides may not be published, posted online, or used in commercial presentations. These content slides were current based on the video production date of 3/29/19.

Topics We Will Touch on Today • Personalized risk stratification models beyond IPSS and IPSSR • Is there a benefit to treating iron overload in transfusion dependent MDS? • Newer strategies for treating lower-risk MDS patients who are transfusion dependent • Immunotherapies in MDS • New strategies for treating high-risk MDS

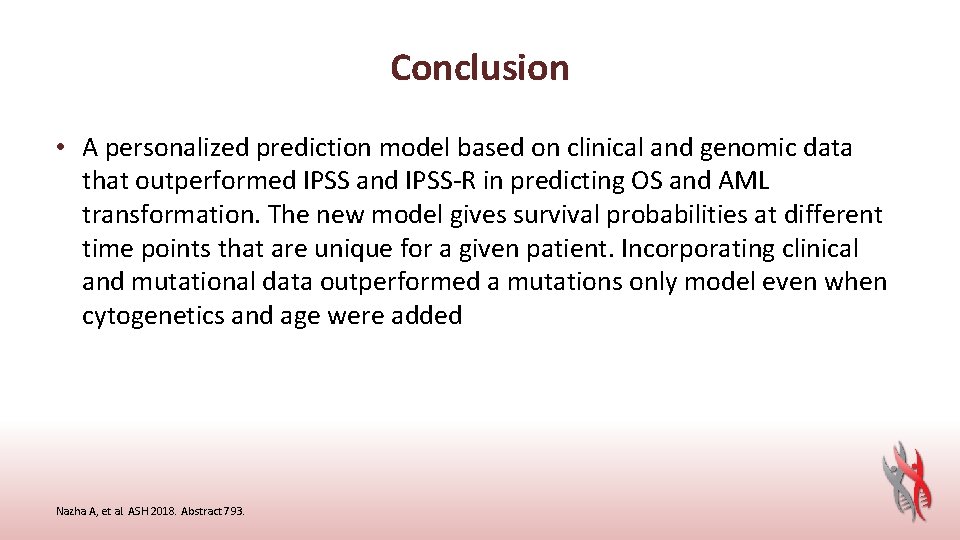
Background • Patients with myelodysplastic syndromes (MDS) have heterogeneous outcomes that can range from months for some patients to decades for others. Although several prognostic scoring systems have been developed to risk stratify MDS patients, survival varies even within discrete categories, which may lead to over- or under-treatment. Deficits in discriminatory power likely derive from analytic approaches or lack of incorporation of molecular data • Here, we developed a model that uses a machine learning approach to analyze genomic and clinical data to provide a personalized overall outcome that is patient-specific Nazha A, et al. ASH 2018. Abstract 793.

Conclusion • A personalized prediction model based on clinical and genomic data that outperformed IPSS and IPSS-R in predicting OS and AML transformation. The new model gives survival probabilities at different time points that are unique for a given patient. Incorporating clinical and mutational data outperformed a mutations only model even when cytogenetics and age were added Nazha A, et al. ASH 2018. Abstract 793.

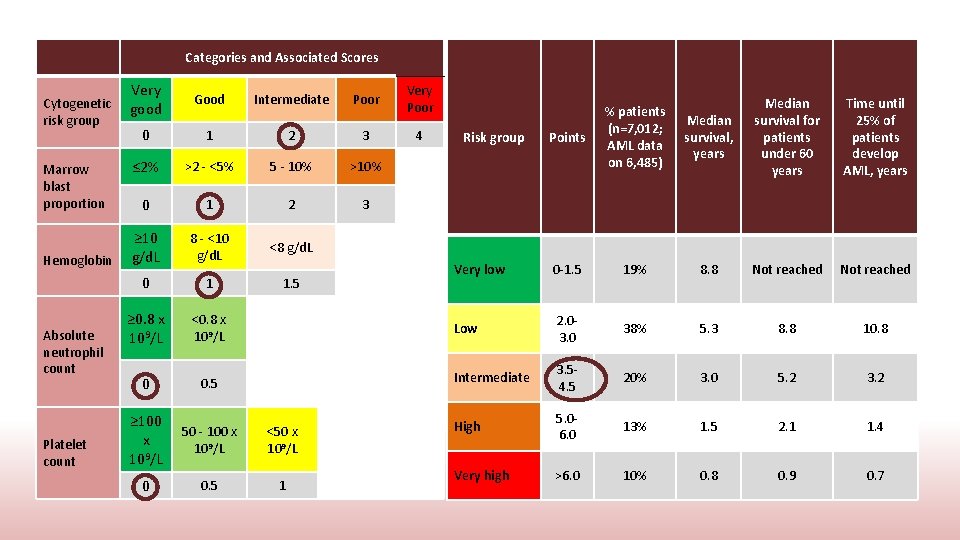
Categories and Associated Scores Very Cytogenetic good risk group Marrow blast proportion Hemoglobin Absolute neutrophil count Platelet count Good Intermediate Poor Very Poor 0 1 2 3 4 ≤ 2% >2 - <5% 5 - 10% >10% 0 1 2 3 ≥ 10 g/d. L 8 - <10 g/d. L <8 g/d. L 0 1 1. 5 ≥ 0. 8 x 109/L <0. 8 x 109/L 0 0. 5 ≥ 100 x 109/L 50 - 100 x 109/L <50 x 109/L 0 0. 5 1 Median survival, years Median survival for patients under 60 years Time until 25% of patients develop AML, years 19% 8. 8 Not reached 2. 03. 0 38% 5. 3 8. 8 10. 8 Intermediate 3. 54. 5 20% 3. 0 5. 2 3. 2 High 5. 06. 0 13% 1. 5 2. 1 1. 4 Very high >6. 0 10% 0. 8 0. 9 0. 7 Points % patients (n=7, 012; AML data on 6, 485) Very low 0 -1. 5 Low Risk group

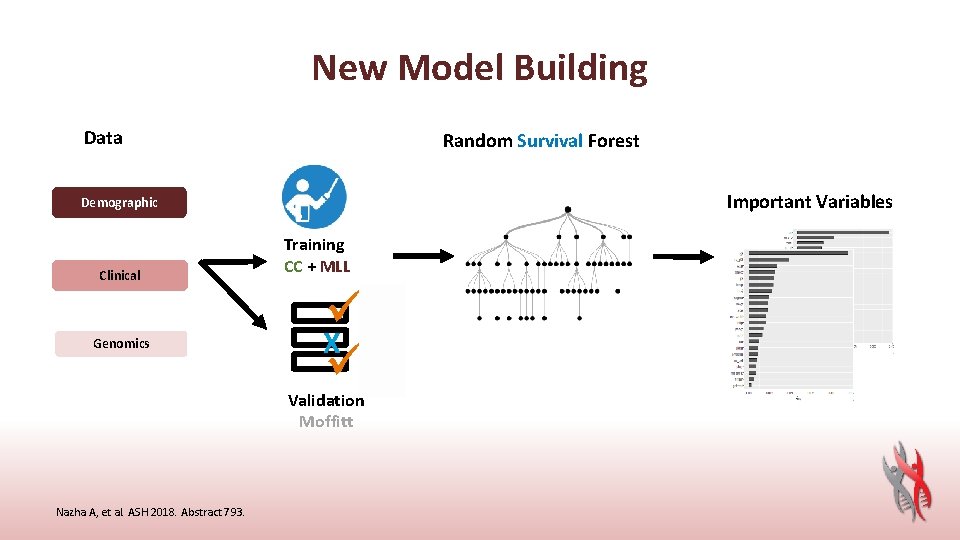
New Model Building Data Random Survival Forest Important Variables Demographic Clinical Genomics Training CC + MLL ü 1 X ü 1 Validation Moffitt Nazha A, et al. ASH 2018. Abstract 793.

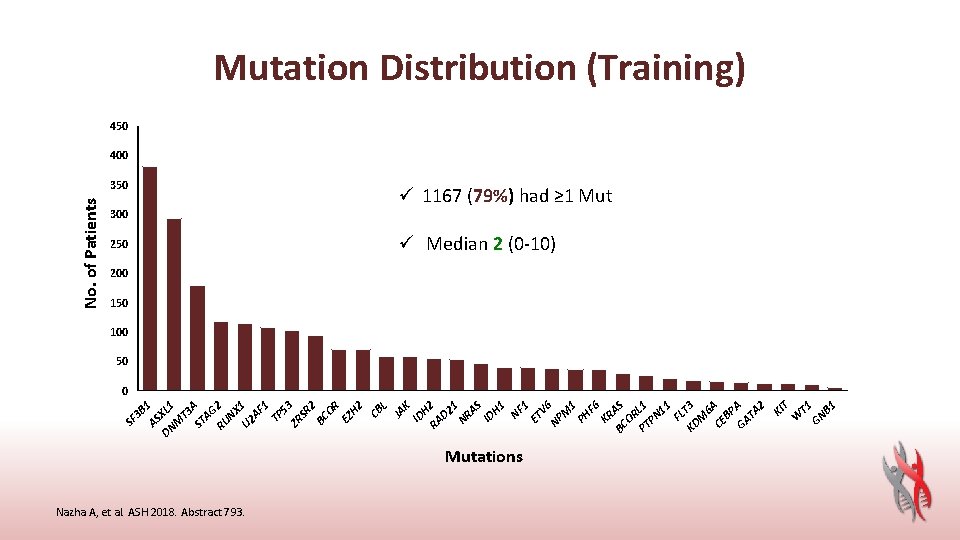
Mutations Nazha A, et al. ASH 2018. Abstract 793. T 1 T B 1 GN W KI KR BC AS OR PT L 1 PN 11 FL KD T 3 M 6 CE A BP GA A TA 2 F 6 PH 1 V 6 M NP 250 1 300 ET 350 NF 3 B 1 AS DN XL 1 M T 3 ST A AG RU 2 NX U 2 1 AF 1 TP 53 ZR SR 2 BC OR EZ H 2 CB L JA K ID H RA 2 D 2 1 NR AS ID H 1 SF No. of Patients Mutation Distribution (Training) 450 400 ü 1167 (79%) had ≥ 1 Mut ü Median 2 (0 -10) 200 150 100 50 0

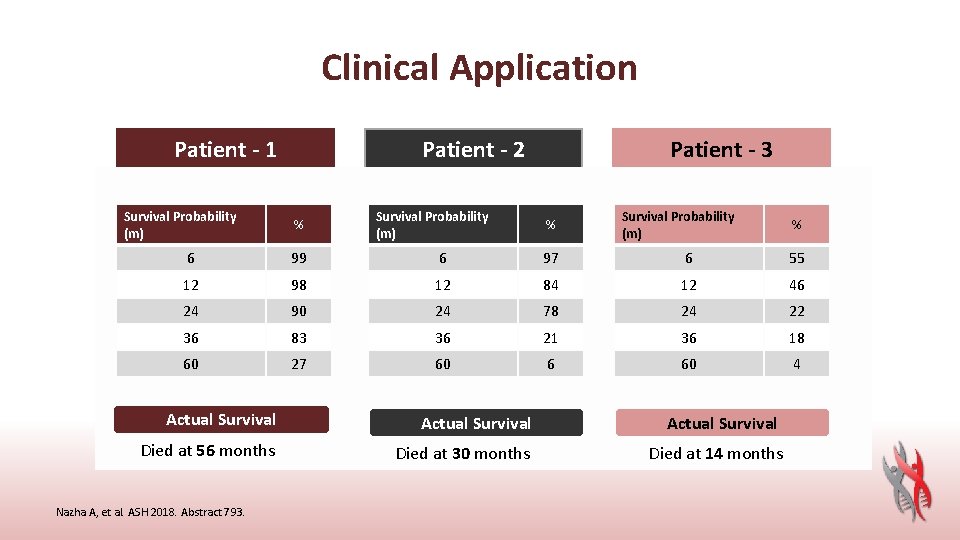
Clinical Application Patient - 1 Survival Probability • Age: (m) • • • WHO: Cyto: 6 BM Blasts: 12 HB: 24 WBC: 36 Platelets: Phenotype: 60 Mutations: 58 y/o% del 5 q 99 del 5 q 2%98 9. 7 90 2. 27 74 83 Primary 27 ASXL 1 Actual Survival Died at 56 months Nazha A, et al. ASH 2018. Abstract 793. Patient - 2 • Survival Probability Age: 71 y/o% (m) • • • WHO: RAEB-1 Cyto: 6 NK 97 BM Blasts: 7%84 12 HB: 8. 3 24 78 WBC: 10. 6 36 Platelets: 10521 Phenotype: Secondary 6 60 Mutations: DNMT 3 A SRSF 2 Actual Survival Died at 30 months Patient - 3 • Age: 71 y/o Survival Probability • (m) WHO: RCMD % • Cyto: +8 6 55 • BM Blasts: 2% • HB: 12 10. 3 46 • WBC: 24 7. 4 22 • Platelets: 120 36 18 • Phenotype: Primary 60 • Mutations: SUZ 12 4 • TET 2 • ASXL 1 Actual Survival Died at 14 months

Safety and Efficacy, Including Event-free Survival, of Deferasirox Versus Placebo in Iron-Overloaded Patients with Low- and Int-1 -Risk Myelodysplastic Syndromes (MDS): Outcomes from the Randomized, Double-Blind TELESTO Study Emanuele Angelucci, 1 Junmin Li, 2 Peter Greenberg, 3 Depei Wu, 4 Ming Hou, 5 Efreen Horacio Montaňo Figueroa, 6 Maria Guadalupe Rodriguez, 7 Xunwei Dong, 8 Jagannath Ghosh, 8 Miguel Izquierdo, 9 and Guillermo Garcia-Manero 10 and Transplant Center, IRCCS Ospedale Policlinico San Martino, Genova, Italy; 2 Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China; 3 Stanford University Medical Center, Stanford, CA, USA; 4 Jiangsu Institute of Hematology, First Affiliated Hospital of Soochow University, Suzhou, China; 5 Department of Hematology, Qilu Hospital, Shandong University, Jinan, China; 6 Department of Hematology, Hospital General de México, Mexico City, Mexico; 7 Department of Hematology, Hospital de Especialidades, Centro Médico Nacional La Raza, IMSS, Mexico City, Mexico; 8 Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; 9 Novartis Pharma AG, Basel, Switzerland; 10 MD Anderson Cancer Center, University of Texas, Houston, TX, USA 1 Hematology

Background and Study Rationale • Although iron chelation therapy (ICT) has been shown to improve outcomes in lower -risk MDS patients, the studies were mainly retrospective analyses and registry studies 1 -6 • However, considerable debate remained on the clinical utility of ICT in this patient population, and the need for a randomized trial has long been recognized 7 Aims The TELESTO (NCT 00940602) study prospectively evaluated event-free survival (EFS) and the safety of ICT with deferasirox versus placebo in patients with low/intermediate (Int)-1 -risk MDS AML=acute myeloid leukemia; RBC=red blood cell 1 Delforge M, et al. Leuk Res. 2014; 38: 557 -563. 2 Leitch HA, et al. Clin Leukemia. 2008; 2: 205 -211. 3 Lyons RM, et al. Leuk Res. 2017; 56: 88 -95. 4 Neukirchen J, et al. Leuk Res. 2012; 36: 1067 -1070. 5 Remacha AF, et al. Ann Hematol. 2015; 94: 779 -787. 6 Rose C, et al. Leuk Res. 2010; 34: 864 -870. 7 Meerpohl JJ, et al. Cochrane Database Syst Rev. 2014: CD 007461.

Primary To evaluate event-free survival (composite endpoint) • Defined as the time from randomization to first documented non-fatal event (worsening cardiac function, hospitalization for congestive heart failure, liver function impairment, liver cirrhosis, transformation to AML), based on review and confirmation by an independent adjudication committee, or death, whichever occurred first Key secondary TELESTO – Study Objectives To assess: • Overall survival • Change in serum ferritin level • Hematologic improvement in terms of erythroid response (based on International MDS Working Group criteria 1) • Change in endocrine function (thyroid and glycemic control) • Safety 1 Cheson BD, et al. Blood. 2006; 108: 419 -425.

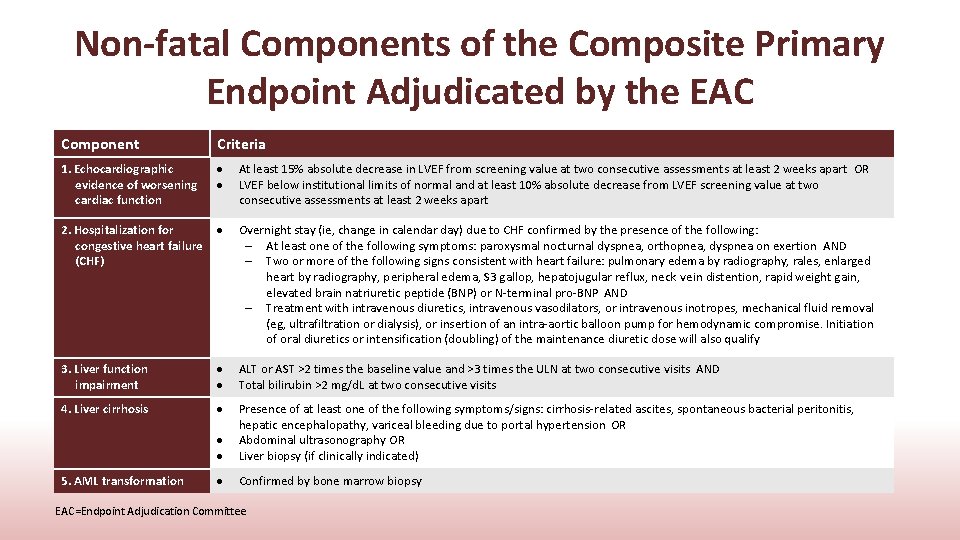
Non-fatal Components of the Composite Primary Endpoint Adjudicated by the EAC Component Criteria 1. Echocardiographic evidence of worsening cardiac function 2. Hospitalization for congestive heart failure (CHF) At least 15% absolute decrease in LVEF from screening value at two consecutive assessments at least 2 weeks apart OR LVEF below institutional limits of normal and at least 10% absolute decrease from LVEF screening value at two consecutive assessments at least 2 weeks apart Overnight stay (ie, change in calendar day) due to CHF confirmed by the presence of the following: – At least one of the following symptoms: paroxysmal nocturnal dyspnea, orthopnea, dyspnea on exertion AND – Two or more of the following signs consistent with heart failure: pulmonary edema by radiography, rales, enlarged heart by radiography, peripheral edema, S 3 gallop, hepatojugular reflux, neck vein distention, rapid weight gain, elevated brain natriuretic peptide (BNP) or N-terminal pro-BNP AND – Treatment with intravenous diuretics, intravenous vasodilators, or intravenous inotropes, mechanical fluid removal (eg, ultrafiltration or dialysis), or insertion of an intra-aortic balloon pump for hemodynamic compromise. Initiation of oral diuretics or intensification (doubling) of the maintenance diuretic dose will also qualify 3. Liver function impairment ALT or AST >2 times the baseline value and >3 times the ULN at two consecutive visits AND Total bilirubin >2 mg/d. L at two consecutive visits 4. Liver cirrhosis Presence of at least one of the following symptoms/signs: cirrhosis-related ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, variceal bleeding due to portal hypertension OR Abdominal ultrasonography OR Liver biopsy (if clinically indicated) Confirmed by bone marrow biopsy 5. AML transformation EAC=Endpoint Adjudication Committee

Key Demographic and Baseline Characteristics Demographic variable Deferasirox (N=149) Placebo (N=76) All patients (N=225) 61. 2 (16. 1) 66 (21– 88) 60. 7 (15. 1) 65 (20– 80) 61. 0 (15. 7) 65 (20– 88) Age category (years), n (%) <50 50–<65 65–<75 ≥ 75 37 (24. 8) 34 (22. 8) 40 (26. 8) 38 (25. 5) 20 (26. 3) 17 (22. 4) 26 (34. 2) 13 (17. 1) 57 (25. 3) 51 (22. 7) 66 (29. 3) 51 (22. 7) Male, n (%) 93 (62. 4) 44 (57. 9) 137 (60. 9) Race, n (%) Caucasian Asian Other 68 (45. 6) 66 (44. 3) 15 (10. 1) 36 (47. 4) 34 (44. 7) 6 (7. 9) 104 (46. 2) 100 (44. 4) 21 (9. 3) MDS risk category (IPSS), n (%) Low Int-1 41 (27. 5) 108 (72. 5) 21 (27. 6) 55 (72. 4) 62 (27. 6) 163 (72. 4) 35 (23. 5) 114 (76. 5) 14 (18. 4) 49 (21. 8) Age, years Mean (SD) Median (range) Prior chelation therapy, n (%) Yes SD=standard deviation

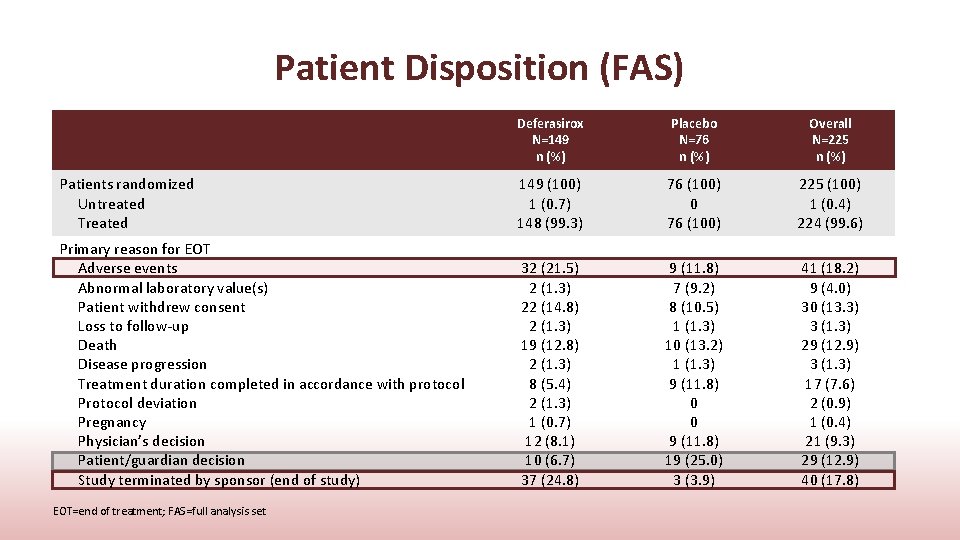
Patient Disposition (FAS) Deferasirox N=149 n (%) Placebo N=76 n (%) Overall N=225 n (%) Patients randomized Untreated Treated 149 (100) 1 (0. 7) 148 (99. 3) 76 (100) 0 76 (100) 225 (100) 1 (0. 4) 224 (99. 6) Primary reason for EOT Adverse events Abnormal laboratory value(s) Patient withdrew consent Loss to follow-up Death Disease progression Treatment duration completed in accordance with protocol Protocol deviation Pregnancy Physician’s decision Patient/guardian decision Study terminated by sponsor (end of study) 32 (21. 5) 2 (1. 3) 22 (14. 8) 2 (1. 3) 19 (12. 8) 2 (1. 3) 8 (5. 4) 2 (1. 3) 1 (0. 7) 12 (8. 1) 10 (6. 7) 37 (24. 8) 9 (11. 8) 7 (9. 2) 8 (10. 5) 1 (1. 3) 10 (13. 2) 1 (1. 3) 9 (11. 8) 0 0 9 (11. 8) 19 (25. 0) 3 (3. 9) 41 (18. 2) 9 (4. 0) 30 (13. 3) 3 (1. 3) 29 (12. 9) 3 (1. 3) 17 (7. 6) 2 (0. 9) 1 (0. 4) 21 (9. 3) 29 (12. 9) 40 (17. 8) EOT=end of treatment; FAS=full analysis set

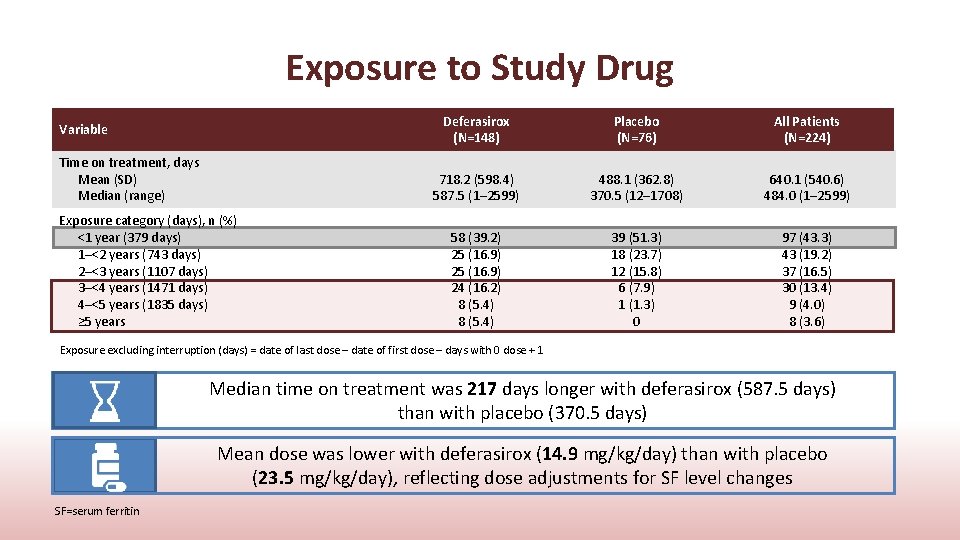
Exposure to Study Drug Variable Time on treatment, days Mean (SD) Median (range) Exposure category (days), n (%) <1 year (379 days) 1–<2 years (743 days) 2–<3 years (1107 days) 3–<4 years (1471 days) 4–<5 years (1835 days) ≥ 5 years Deferasirox (N=148) Placebo (N=76) All Patients (N=224) 718. 2 (598. 4) 587. 5 (1– 2599) 488. 1 (362. 8) 370. 5 (12– 1708) 640. 1 (540. 6) 484. 0 (1– 2599) 58 (39. 2) 25 (16. 9) 24 (16. 2) 8 (5. 4) 39 (51. 3) 18 (23. 7) 12 (15. 8) 6 (7. 9) 1 (1. 3) 0 97 (43. 3) 43 (19. 2) 37 (16. 5) 30 (13. 4) 9 (4. 0) 8 (3. 6) Exposure excluding interruption (days) = date of last dose – date of first dose – days with 0 dose + 1 Median time on treatment was 217 days longer with deferasirox (587. 5 days) than with placebo (370. 5 days) Mean dose was lower with deferasirox (14. 9 mg/kg/day) than with placebo (23. 5 mg/kg/day), reflecting dose adjustments for SF level changes SF=serum ferritin

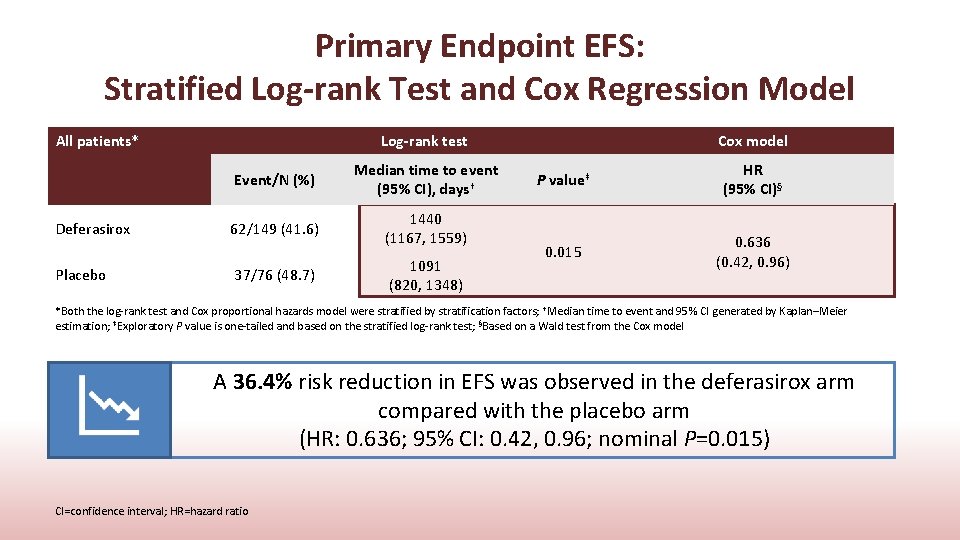
Primary Endpoint EFS: Stratified Log-rank Test and Cox Regression Model All patients* Deferasirox Placebo Log-rank test Event/N (%) Median time to event (95% CI), days† 62/149 (41. 6) 1440 (1167, 1559) 37/76 (48. 7) 1091 (820, 1348) Cox model P value‡ HR (95% CI)§ 0. 015 0. 636 (0. 42, 0. 96) *Both the log-rank test and Cox proportional hazards model were stratified by stratification factors; †Median time to event and 95% CI generated by Kaplan–Meier estimation; ‡Exploratory P value is one-tailed and based on the stratified log-rank test; §Based on a Wald test from the Cox model A 36. 4% risk reduction in EFS was observed in the deferasirox arm compared with the placebo arm (HR: 0. 636; 95% CI: 0. 42, 0. 96; nominal P=0. 015) CI=confidence interval; HR=hazard ratio

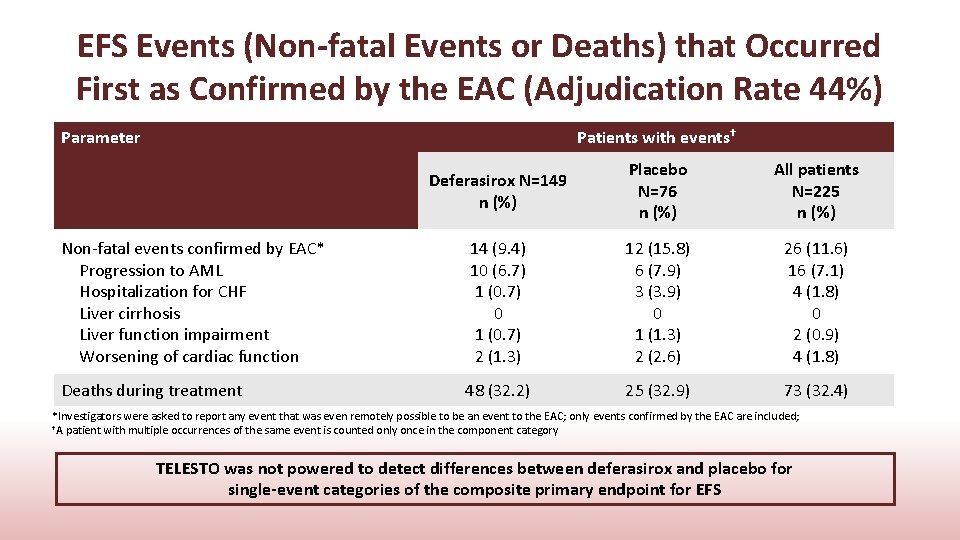
EFS Events (Non-fatal Events or Deaths) that Occurred First as Confirmed by the EAC (Adjudication Rate 44%) Parameter Patients with events† Deferasirox N=149 n (%) Placebo N=76 n (%) All patients N=225 n (%) Non-fatal events confirmed by EAC* Progression to AML Hospitalization for CHF Liver cirrhosis Liver function impairment Worsening of cardiac function 14 (9. 4) 10 (6. 7) 1 (0. 7) 0 1 (0. 7) 2 (1. 3) 12 (15. 8) 6 (7. 9) 3 (3. 9) 0 1 (1. 3) 2 (2. 6) 26 (11. 6) 16 (7. 1) 4 (1. 8) 0 2 (0. 9) 4 (1. 8) Deaths during treatment 48 (32. 2) 25 (32. 9) 73 (32. 4) *Investigators were asked to report any event that was even remotely possible to be an event to the EAC; only events confirmed by the EAC are included; †A patient with multiple occurrences of the same event is counted only once in the component category TELESTO was not powered to detect differences between deferasirox and placebo for single-event categories of the composite primary endpoint for EFS

Summary of Overall Survival All patients* Log-rank test Cox model Event/N (%) Median time (95% CI), days† Deferasirox 57/149 (38. 3) 1907 (1440, NE) Placebo 33/76 (43. 4) 1509 (1095, 1804) P value‡ Hazard ratio (95% CI)§ 0. 200 0. 832 (0. 54, 1. 28) *Both log-rank test and Cox proportional hazards model were stratified by stratification factors; †Median time to event and 95% CI generated by Kaplan–Meier estimation; ‡Exploratory P value is one-tailed and based on the stratified log-rank test; §Based on a Wald test from the Cox model Median OS was prolonged by 398 days with deferasirox vs placebo Probability of overall survival (%) 100 80 Randomized treatment Deferasirox Placebo Censored 60 40 20 0 Subjects Events Deferasirox 149 57 Placebo 76 33 HR (95% CI) = 0. 832 (0. 540, 1. 279) 0 No. of patients still at risk Deferasirox Placebo NE=not evaluable 149 76 364 113 60 728 91 45 Median OS (days) (95% CI) 1907 1509 (1440, NE) (1095, 1804) 1092 76 33 1456 Time (days) 40 18 Following study drug discontinuation 52. 1% of placebo patients started ICT 1820 2184 2548 2912 20 4 7 1 1 0 0

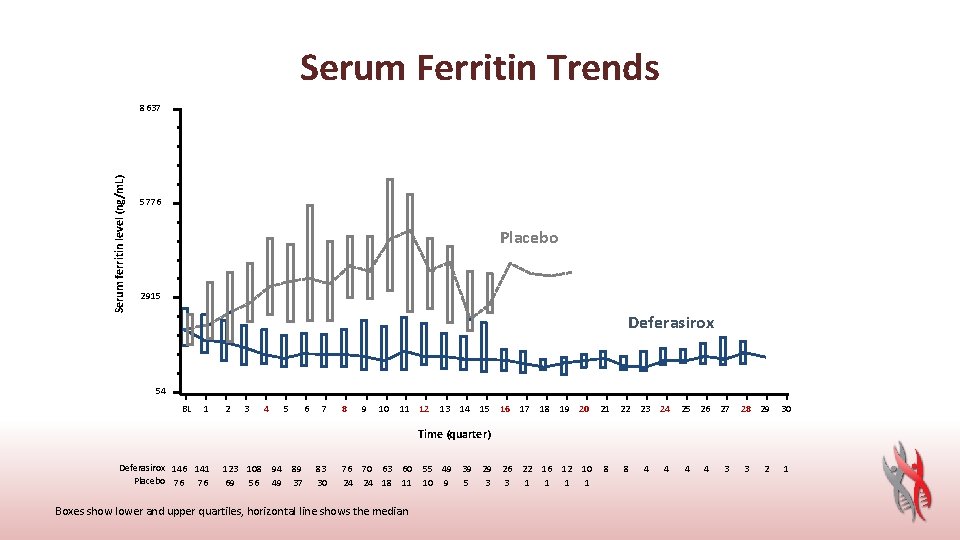
Serum Ferritin Trends Serum ferritin level (ng/m. L) 8637 5776 Placebo 2915 Deferasirox 54 BL 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 Time (quarter) Deferasirox 146 141 Placebo 76 76 123 108 94 69 56 49 89 37 83 30 76 70 63 60 24 24 18 11 Boxes show lower and upper quartiles, horizontal line shows the median 55 49 39 29 10 9 5 3 26 22 16 12 10 3 1 1 8 8 4 4 3 3 2 1

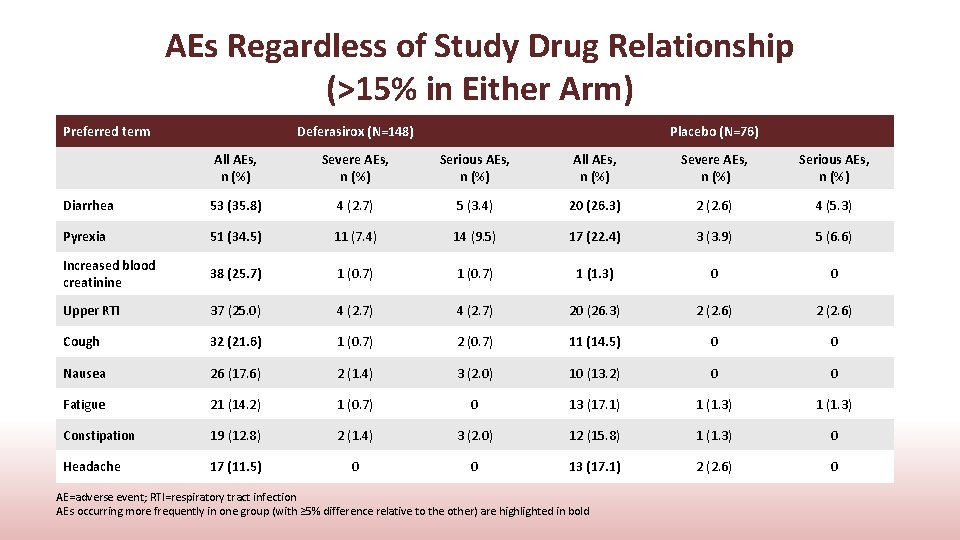
AEs Regardless of Study Drug Relationship (>15% in Either Arm) Preferred term Deferasirox (N=148) Placebo (N=76) All AEs, n (%) Severe AEs, n (%) Serious AEs, n (%) Diarrhea 53 (35. 8) 4 (2. 7) 5 (3. 4) 20 (26. 3) 2 (2. 6) 4 (5. 3) Pyrexia 51 (34. 5) 11 (7. 4) 14 (9. 5) 17 (22. 4) 3 (3. 9) 5 (6. 6) Increased blood creatinine 38 (25. 7) 1 (0. 7) 1 (1. 3) 0 0 Upper RTI 37 (25. 0) 4 (2. 7) 20 (26. 3) 2 (2. 6) Cough 32 (21. 6) 1 (0. 7) 2 (0. 7) 11 (14. 5) 0 0 Nausea 26 (17. 6) 2 (1. 4) 3 (2. 0) 10 (13. 2) 0 0 Fatigue 21 (14. 2) 1 (0. 7) 0 13 (17. 1) 1 (1. 3) Constipation 19 (12. 8) 2 (1. 4) 3 (2. 0) 12 (15. 8) 1 (1. 3) 0 Headache 17 (11. 5) 0 0 13 (17. 1) 2 (2. 6) 0 AE=adverse event; RTI=respiratory tract infection AEs occurring more frequently in one group (with ≥ 5% difference relative to the other) are highlighted in bold

Summary • TELESTO is the first prospective, randomized study of ICT in patients with Low-/Int-1 -risk MDS and iron overload • Treatment with deferasirox led to longer EFS compared with placebo • Exposure-adjusted AEs were similar in the two arms with the exception of nonsevere increases in serum creatinine, with no new safety signals • Considering the current treatment landscape, it is unlikely that a similar randomized trial will be performed TELESTO provides evidence on the clinical benefit of ICT in lower-risk MDS patients with iron overload

The MEDALIST Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo. Controlled Study of Luspatercept to Treat Patients with Very Low-, or Intermediate-Risk Myelodysplastic Syndromes (MDS) Associated Anemia with Ring Sideroblasts (RS) Who Require Red Blood Cell (RBC) Transfusions Pierre Fenaux, Uwe Platzbecker, Ghulam J. Mufti, Guillermo Garcia-Manero, Rena Buckstein, Valeria Santini, María Díez-Campelo, Carlo Finelli, Mario Cazzola, Osman Ilhan, Mikkael A. Sekeres, José F. Falantes, Beatriz Arrizabalaga, Flavia Salvi, Valentina Giai, Paresh Vyas, David Bowen, Dominik Selleslag, Amy E. De. Zern, Joseph G. Jurcic, Ulrich Germing, Katharina S. Götze, Bruno Quesnel, Odile Beyne-Rauzy, Thomas Cluzeau, Maria Teresa Voso, Dominiek Mazure, Edo Vellenga, Peter L. Greenberg, Eva Hellström. Lindberg, Amer M. Zeidan, Abderrahmane Laadem, Aziz Benzohra, Jennie Zhang, Anita Rampersad, Peter G. Linde, Matthew L. Sherman, Rami S. Komrokji and Alan F. List • Patients with lower-risk (LR)a transfusion-dependent MDS have a poorer prognosis, with greater risk of progression to AML and inferior overall survival compared to transfusion-independent MDS patients • RBC transfusion-dependent LR, non-del(5 q) MDS patients have a transient response to ESAs, with an attendant risk of iron overload and secondary organ complications • Few treatment options exist for LR MDS patients who are either refractory to or become unresponsive to erythropoiesis-stimulating agents (ESAs)1 a IPSS-R-defined criteria. ESA=erythropoiesis-stimulating agent; IPSS-R=Revised International Prognostic Scoring System; TGF-β=transforming growth factor-beta 2013; 121: 4280 -4286. 1 Fenaux P, Ades L. Blood.

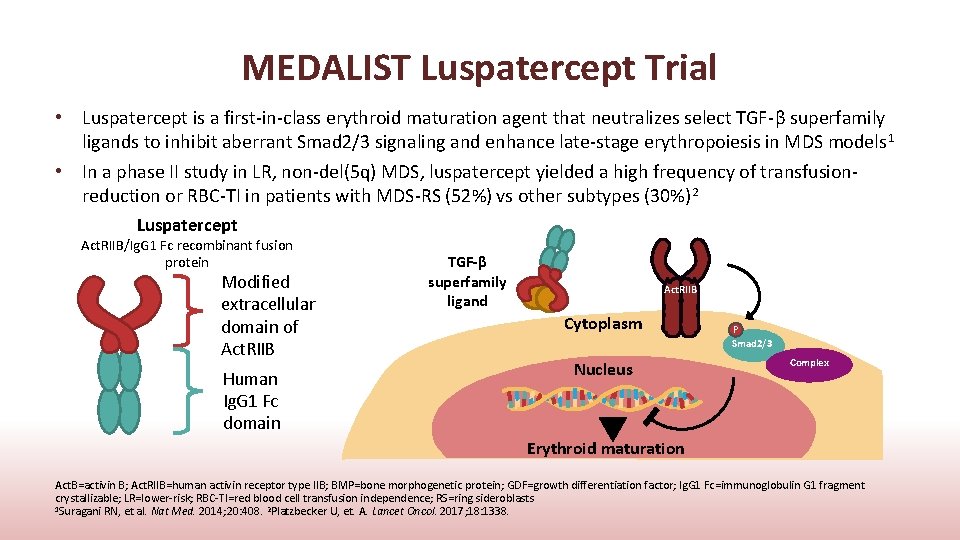
MEDALIST Luspatercept Trial • Luspatercept is a first-in-class erythroid maturation agent that neutralizes select TGF-β superfamily ligands to inhibit aberrant Smad 2/3 signaling and enhance late-stage erythropoiesis in MDS models 1 • In a phase II study in LR, non-del(5 q) MDS, luspatercept yielded a high frequency of transfusionreduction or RBC-TI in patients with MDS-RS (52%) vs other subtypes (30%) 2 Luspatercept Act. RIIB/Ig. G 1 Fc recombinant fusion protein Modified extracellular domain of Act. RIIB Human Ig. G 1 Fc domain TGF-β superfamily ligand Act. RIIB Cytoplasm Nucleus P Smad 2/3 Complex Erythroid maturation Act. B=activin B; Act. RIIB=human activin receptor type IIB; BMP=bone morphogenetic protein; GDF=growth differentiation factor; Ig. G 1 Fc=immunoglobulin G 1 fragment crystallizable; LR=lower-risk; RBC-TI=red blood cell transfusion independence; RS=ring sideroblasts 1 Suragani RN, et al. Nat Med. 2014; 20: 408. 2 Platzbecker U, et. A. Lancet Oncol. 2017; 18: 1338.

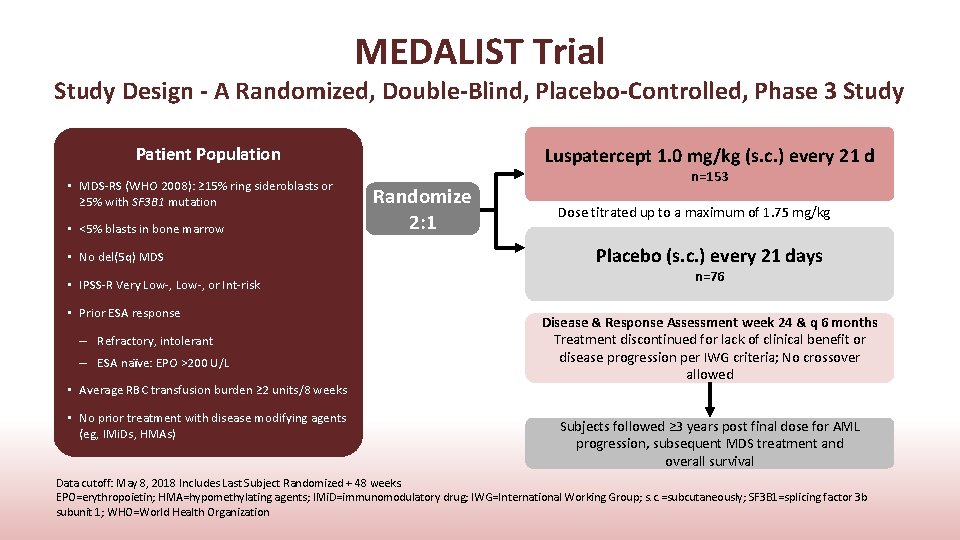
MEDALIST Trial Study Design - A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study Patient Population • MDS-RS (WHO 2008): ≥ 15% ring sideroblasts or ≥ 5% with SF 3 B 1 mutation • <5% blasts in bone marrow • No del(5 q) MDS • IPSS-R Very Low-, or Int-risk • Prior ESA response – Refractory, intolerant – ESA naïve: EPO >200 U/L • Average RBC transfusion burden ≥ 2 units/8 weeks • No prior treatment with disease modifying agents (eg, IMi. Ds, HMAs) Luspatercept 1. 0 mg/kg (s. c. ) every 21 d Randomize 2: 1 n=153 Dose titrated up to a maximum of 1. 75 mg/kg Placebo (s. c. ) every 21 days n=76 Disease & Response Assessment week 24 & q 6 months Treatment discontinued for lack of clinical benefit or disease progression per IWG criteria; No crossover allowed Subjects followed ≥ 3 years post final dose for AML progression, subsequent MDS treatment and overall survival Data cutoff: May 8, 2018 Includes Last Subject Randomized + 48 weeks. EPO=erythropoietin; HMA=hypomethylating agents; IMi. D=immunomodulatory drug; IWG=International Working Group; s. c. =subcutaneously; SF 3 B 1=splicing factor 3 b subunit 1; WHO=World Health Organization

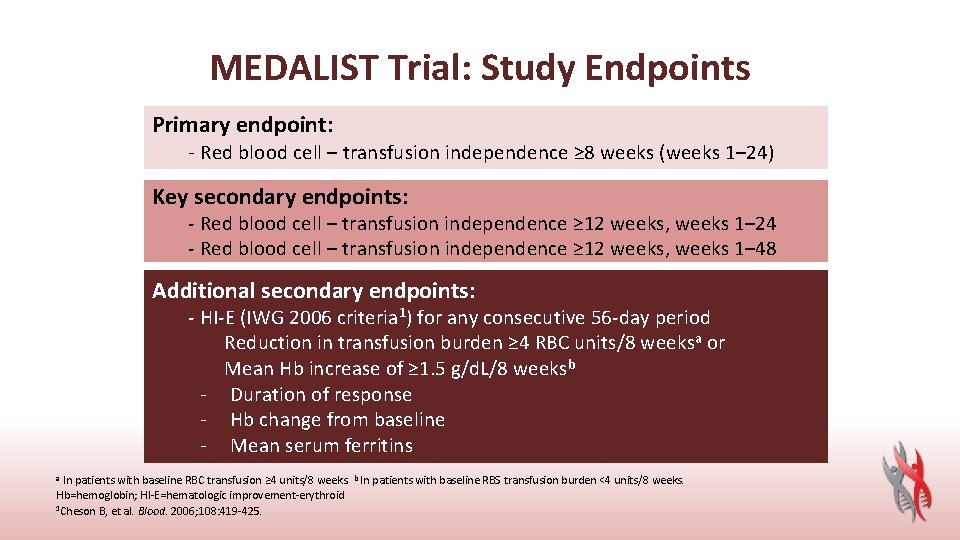
MEDALIST Trial: Study Endpoints Primary endpoint: - Red blood cell – transfusion independence ≥ 8 weeks (weeks 1– 24) Key secondary endpoints: - Red blood cell – transfusion independence ≥ 12 weeks, weeks 1– 24 - Red blood cell – transfusion independence ≥ 12 weeks, weeks 1– 48 Additional secondary endpoints: - HI-E (IWG 2006 criteria 1) for any consecutive 56 -day period Reduction in transfusion burden ≥ 4 RBC units/8 weeksa or Mean Hb increase of ≥ 1. 5 g/d. L/8 weeksb - Duration of response - Hb change from baseline - Mean serum ferritins a In patients with baseline RBC transfusion ≥ 4 units/8 weeks. b In patients with baseline RBS transfusion burden <4 units/8 weeks. Hb=hemoglobin; HI-E=hematologic improvement-erythroid 1 Cheson B, et al. Blood. 2006; 108: 419 -425.

MEDALIST Trial Demographics and Baseline Disease Characteristics Characteristic Age, median (range), years Male, n (%) Time since original MDS diagnosis, median (range), months WHO Classification RCMDa or RCMD-RS, n (%) RBC transfusion burden, median (range), units/8 weeks a ≥ 6 units/8 weeks, n (%) <6 units/8 weeks, n (%) Pretransfusion Hb, median (range), g/d. L IPSS-R risk category stratificationb Very Low, n (%) Intermediate, n (%) SF 3 B 1 mutation, n (%) Serum EPO <200 U/L, n (%) ≥ 200 U/L, n (%) Luspatercept (n=153) Placebo (n=76) 71 (40– 95) 94 (61. 4) 44. 0 (3– 421) 72 (26– 91) 50 (65. 8) 36. 1 (4– 193) 145 (94. 8) 5 (1– 15) 66 (43. 1) 87 (56. 9) 7. 6 (6– 10) 74 (97. 4) 5 (2– 20) 33 (43. 4) 43 (56. 6) 7. 6 (5– 9) 127 (83. 0) 25 (16. 3) 141 (92. 2) 63 (82. 9) 13 (17. 1) 65 (85. 5)c 88 (57. 5) c 64 (41. 8)c 50 (65. 8) 26 (34. 2) a In the 16 weeks prior to randomization. b 1 (0. 7%) patient in the luspatercept arm classified as IPSS-R High. c Data was missing for 1 patient. RARS=refractory anemia with ring sideroblasts; RCMD=refractory cytopenia with multilineage dysplasia; RCMD-RS=RCMD with ring sideroblasts

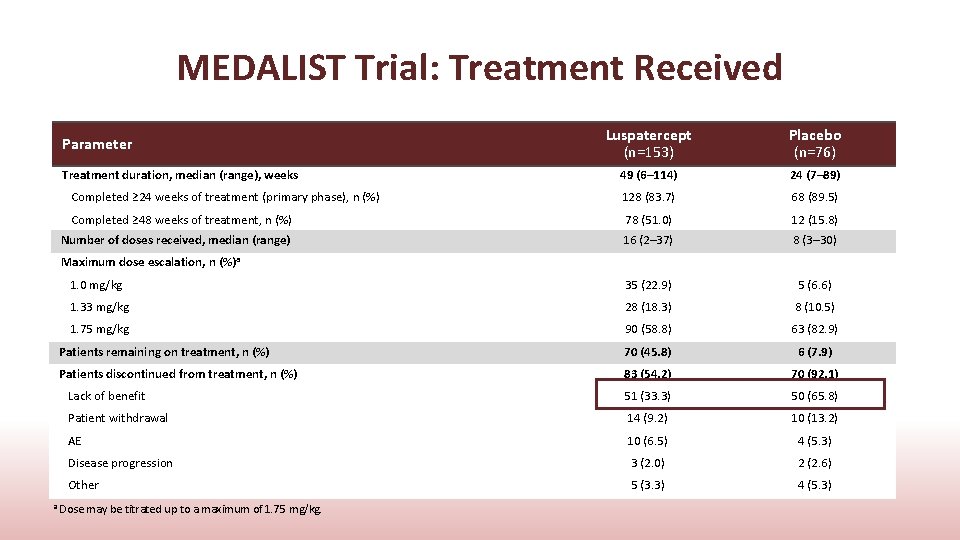
MEDALIST Trial: Treatment Received Luspatercept (n=153) Placebo (n=76) 49 (6– 114) 24 (7– 89) Completed ≥ 24 weeks of treatment (primary phase), n (%) 128 (83. 7) 68 (89. 5) Completed ≥ 48 weeks of treatment, n (%) 78 (51. 0) 12 (15. 8) Number of doses received, median (range) 16 (2– 37) 8 (3– 30) 1. 0 mg/kg 35 (22. 9) 5 (6. 6) 1. 33 mg/kg 28 (18. 3) 8 (10. 5) 1. 75 mg/kg 90 (58. 8) 63 (82. 9) Patients remaining on treatment, n (%) 70 (45. 8) 6 (7. 9) Patients discontinued from treatment, n (%) 83 (54. 2) 70 (92. 1) Lack of benefit 51 (33. 3) 50 (65. 8) Patient withdrawal 14 (9. 2) 10 (13. 2) AE 10 (6. 5) 4 (5. 3) Disease progression 3 (2. 0) 2 (2. 6) Other 5 (3. 3) 4 (5. 3) Parameter Treatment duration, median (range), weeks Maximum dose escalation, n (%)a a Dose may be titrated up to a maximum of 1. 75 mg/kg.

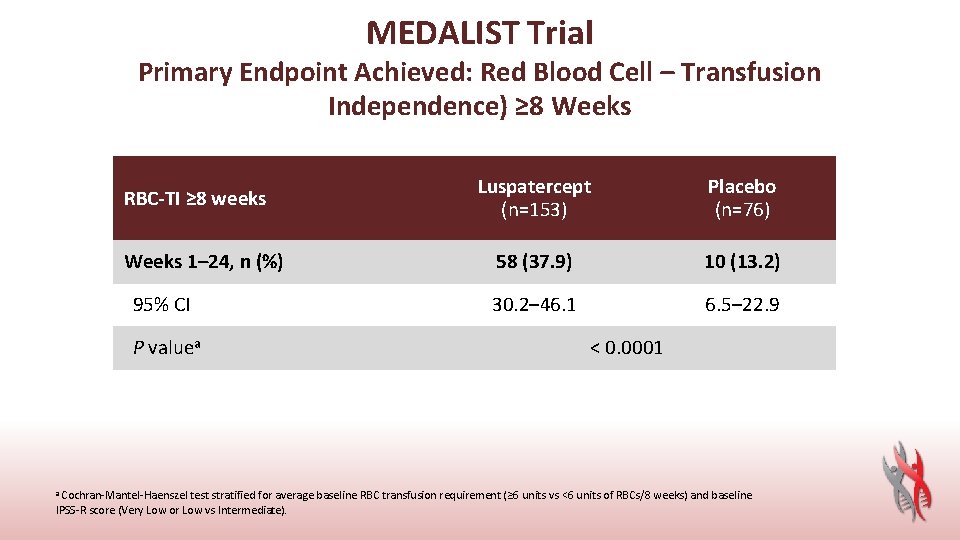
MEDALIST Trial Primary Endpoint Achieved: Red Blood Cell – Transfusion Independence) ≥ 8 Weeks RBC-TI ≥ 8 weeks Weeks 1– 24, n (%) 95% CI P valuea Luspatercept (n=153) Placebo (n=76) 58 (37. 9) 10 (13. 2) 30. 2– 46. 1 6. 5– 22. 9 < 0. 0001 a Cochran-Mantel-Haenszel test stratified for average baseline RBC transfusion requirement (≥ 6 units vs <6 units of RBCs/8 weeks) and baseline IPSS-R score (Very Low or Low vs Intermediate).

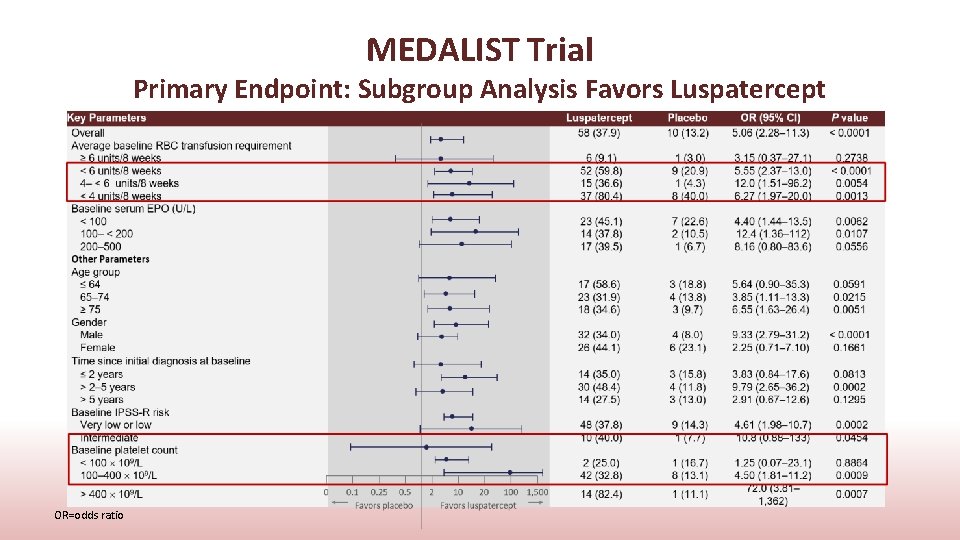
MEDALIST Trial Primary Endpoint: Subgroup Analysis Favors Luspatercept OR=odds ratio

MEDALIST Trial Key Secondary Endpoints: Red Blood Cell – Transfusion Independence ≥ 12 Weeks RBC-TI ≥ 12 weeks Luspatercept (n=153) Placebo (n=76) Weeks 1– 24, n (%) 43 (28. 1) 6 (7. 9) 21. 14– 35. 93 2. 95– 16. 40 95% CI P valuea Weeks 1– 48, n (%) 95% CI P valuea 0. 0002 51 (33. 3) 9 (11. 8) 25. 93– 41. 40 5. 56– 21. 29 0. 0003 a Cochran-Mantel-Haenszel test stratified for average baseline RBC transfusion requirement (≥ 6 units vs <6 units of RBCs/8 weeks) and baseline IPSS-R score (Very Low or Low vs Intermediate).

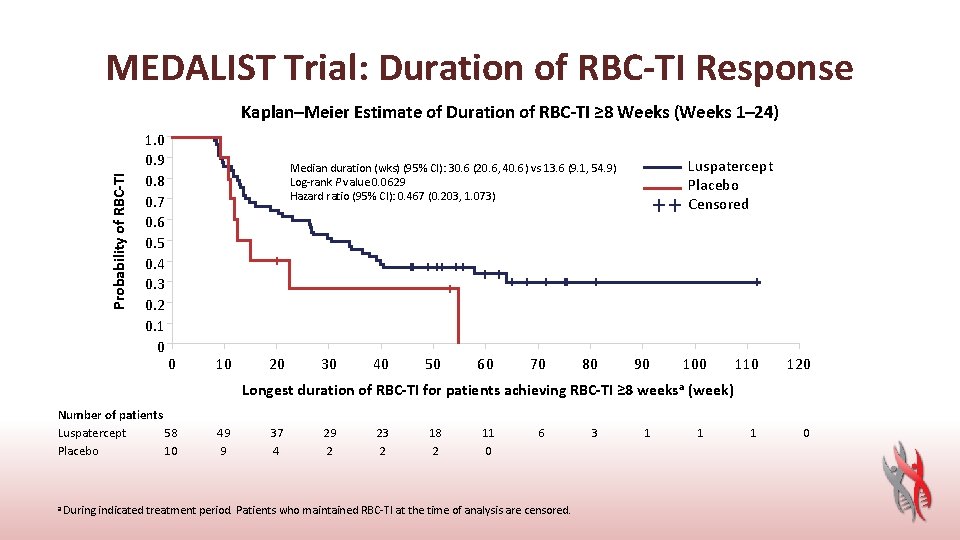
MEDALIST Trial: Duration of RBC-TI Response Probability of RBC-TI Kaplan–Meier Estimate of Duration of RBC-TI ≥ 8 Weeks (Weeks 1– 24) 1. 0 0. 9 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 Luspatercept Placebo Censored Median duration (wks) (95% CI): 30. 6 (20. 6, 40. 6) vs 13. 6 (9. 1, 54. 9) Log-rank P value 0. 0629 Hazard ratio (95% CI): 0. 467 (0. 203, 1. 073) 0 10 20 30 40 50 60 70 80 90 100 110 120 1 0 Longest duration of RBC-TI for patients achieving RBC-TI ≥ 8 weeks a (week) Number of patients Luspatercept 58 Placebo 10 49 9 37 4 29 2 23 2 18 2 11 0 6 a During indicated treatment period. Patients who maintained RBC-TI at the time of analysis are censored. 3 1 1

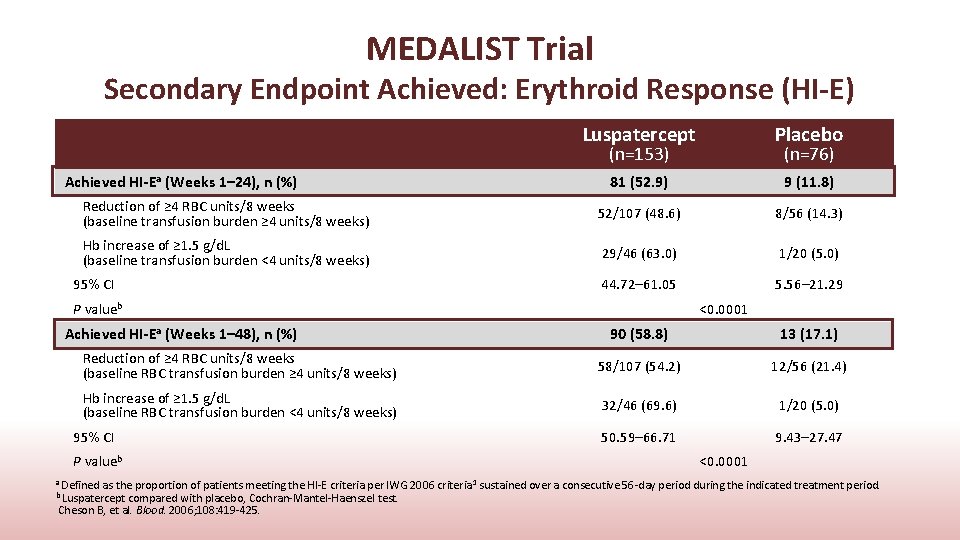
MEDALIST Trial Secondary Endpoint Achieved: Erythroid Response (HI-E) Luspatercept Placebo 81 (52. 9) 9 (11. 8) Reduction of ≥ 4 RBC units/8 weeks (baseline transfusion burden ≥ 4 units/8 weeks) 52/107 (48. 6) 8/56 (14. 3) Hb increase of ≥ 1. 5 g/d. L (baseline transfusion burden <4 units/8 weeks) 29/46 (63. 0) 1/20 (5. 0) 44. 72– 61. 05 5. 56– 21. 29 (n=153) Achieved HI-Ea (Weeks 1– 24), n (%) 95% CI P valueb Achieved HI-Ea (Weeks 1– 48), n (%) (n=76) <0. 0001 90 (58. 8) 13 (17. 1) Reduction of ≥ 4 RBC units/8 weeks (baseline RBC transfusion burden ≥ 4 units/8 weeks) 58/107 (54. 2) 12/56 (21. 4) Hb increase of ≥ 1. 5 g/d. L (baseline RBC transfusion burden <4 units/8 weeks) 32/46 (69. 6) 1/20 (5. 0) 50. 59– 66. 71 9. 43– 27. 47 95% CI P valueb <0. 0001 a Defined as the proportion of patients meeting the HI-E criteria per IWG 2006 criteria 1 sustained over a consecutive 56 -day period during the indicated treatment period. b Luspatercept compared with placebo, Cochran-Mantel-Haenszel test. Cheson B, et al. Blood. 2006; 108: 419 -425.

MEDALIST Trial: Safety Summary Luspatercept Placebo 150 (98. 0) 70 (92. 1) Patients with ≥ 1 suspected related TEAE 67 (43. 8) 27 (35. 5) Patients with ≥ 1 serious TEAE 48 (31. 4) 23 (30. 3) Patients with ≥ 1 Grade 3 or 4 TEAE 65 (42. 5) 34 (44. 7) 5 (3. 3) 4 (5. 3) 13 (8. 5) 6 (7. 9) (n=153) Patients with ≥ 1 TEAE, n (%) Patients with TEAEs leading to deatha Patients with ≥ 1 TEAE causing discontinuation, n (%) (n=76) a In luspatercept arm: sepsis (2), multiple organ dysfunction syndrome, renal failure, hemorrhagic shock; in placebo arm sepsis, urosepsis, general physical health deterioration, respiratory failure TEAE=treatment-emergent adverse event

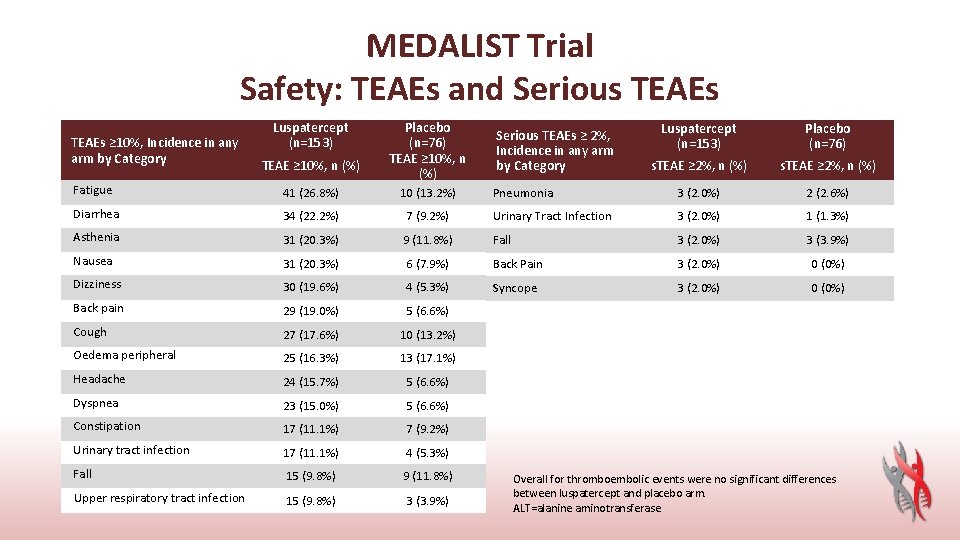
MEDALIST Trial Safety: TEAEs and Serious TEAEs ≥ 10%, Incidence in any arm by Category Luspatercept (n=153) TEAE ≥ 10%, n (%) Placebo (n=76) TEAE ≥ 10%, n (%) Luspatercept (n=153) Placebo (n=76) s. TEAE ≥ 2%, n (%) Pneumonia 3 (2. 0%) 2 (2. 6%) Serious TEAEs ≥ 2%, Incidence in any arm by Category Fatigue 41 (26. 8%) 10 (13. 2%) Diarrhea 34 (22. 2%) 7 (9. 2%) Urinary Tract Infection 3 (2. 0%) 1 (1. 3%) Asthenia 31 (20. 3%) 9 (11. 8%) Fall 3 (2. 0%) 3 (3. 9%) Nausea 31 (20. 3%) 6 (7. 9%) Back Pain 3 (2. 0%) 0 (0%) Dizziness 30 (19. 6%) 4 (5. 3%) Syncope 3 (2. 0%) 0 (0%) Back pain 29 (19. 0%) 5 (6. 6%) Cough 27 (17. 6%) 10 (13. 2%) Oedema peripheral 25 (16. 3%) 13 (17. 1%) Headache 24 (15. 7%) 5 (6. 6%) Dyspnea 23 (15. 0%) 5 (6. 6%) Constipation 17 (11. 1%) 7 (9. 2%) Urinary tract infection 17 (11. 1%) 4 (5. 3%) Fall 15 (9. 8%) 9 (11. 8%) Upper respiratory tract infection 15 (9. 8%) 3 (3. 9%) Overall for thromboembolic events were no significant differences between luspatercept and placebo arm. ALT=alanine aminotransferase

MEDALIST Trial: Conclusions • Luspatercept treatment was well tolerated and yielded a significantly higher proportion of patients who achieved RBC-TI or HI-E (major RBC transfusion reduction, Hb increase) compared with placebo in LR-MDSRS patients (P<0. 0001) • Erythroid responses are durable, with 40% of patients achieving RBC-TI sustained at 12 months of treatment • Luspatercept is a promising novel therapy for the treatment of patients with lower-risk MDS-RS with RBC transfusion-dependent anemia IPSS-R=Revised International Scoring System

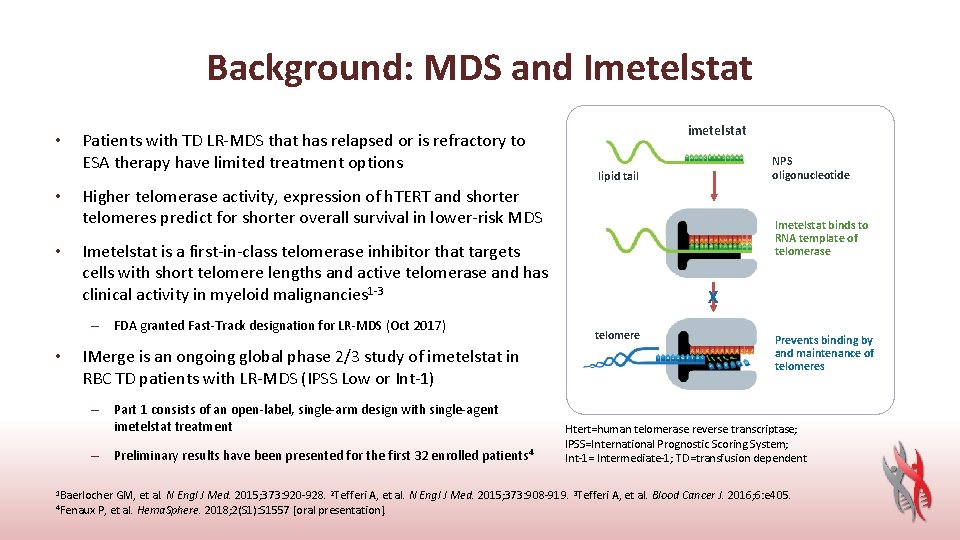
Background: MDS and Imetelstat • • • Patients with TD LR-MDS that has relapsed or is refractory to ESA therapy have limited treatment options NPS oligonucleotide lipid tail Higher telomerase activity, expression of h. TERT and shorter telomeres predict for shorter overall survival in lower-risk MDS Imetelstat binds to RNA template of telomerase Imetelstat is a first-in-class telomerase inhibitor that targets cells with short telomere lengths and active telomerase and has clinical activity in myeloid malignancies 1 -3 – FDA granted Fast-Track designation for LR-MDS (Oct 2017) • imetelstat IMerge is an ongoing global phase 2/3 study of imetelstat in RBC TD patients with LR-MDS (IPSS Low or Int-1) – Part 1 consists of an open-label, single-arm design with single-agent imetelstat treatment – Preliminary results have been presented for the first 32 enrolled patients 4 1 Baerlocher GM, et al. N X telomere Prevents binding by and maintenance of telomeres Htert=human telomerase reverse transcriptase; IPSS=International Prognostic Scoring System; Int-1= Intermediate-1; TD=transfusion dependent Engl J Med. 2015; 373: 920 -928. 2 Tefferi A, et al. N Engl J Med. 2015; 373: 908 -919. 3 Tefferi A, et al. Blood Cancer J. 2016; 6: e 405. 4 Fenaux P, et al. Hema. Sphere. 2018; 2(S 1): S 1557 [oral presentation].

Imetelstat Treatment Leads to Durable Transfusion Independence in RBC Transfusion-Dependent, Non-Del(5 q) Lower Risk MDS Relapsed/Refractory to Erythropoiesis-Stimulating Agent Who Are Lenalidomide and HMA Naive David P. Steensma, MD 1, Uwe Platzbecker, MD 2, Koen Van Eygen, MD 3, Azra Raza, MD 4, Valeria Santini, MD 5, Ulrich Germing, MD, Ph. D 6, Patricia Font, MD 7, Irina Samarina, MD 8, Maria Díez-Campelo, MD, Ph. D 9, Sylvain Thepot, MD 10, Edo Vellenga, MD 11, Mrinal M. Patnaik, MD, MBBS 12, Jun Ho Jang, MD, Ph. D 13, Jacqueline Bussolari, Ph. D 14, Laurie Sherman, BSN 15, Libo Sun, Ph. D 14, Helen Varsos, MS, RPh 14, Esther Rose, MD 14 and Pierre Fenaux, MD, Ph. D 16 1 Dana-Farber Cancer Institute, Boston, MA; 2 Department of Medicine, University Hospital Carl Gustav Carus, TU Dresden, Germany; 3 Algemeen Ziekenhuis Groeninge, Kortrijk, Belgium; 4 Columbia University Medical Center, New York, NY; 5 MDS Unit, AOU Careggi-University of Florence, Italy; 6 Klinik für Hämatologie, Onkologie and Klinische Immunologie, Universitätsklinik Düsseldorf, Heinrich-Heine-Universität, Düsseldorf, Germany; 7 Department of Hematology, Hospital General Universitario Gregorio Marañon, Madrid, Spain; 8 Emergency Hospital of Dzerzhinsk, Nizhny Novgorod, Russian Federation; 9 Hematology Department, The University Hospital of Salamanca, Spain; 10 CHU Angers, France; 11 Department of Hematology, University Medical Center Groningen, Netherlands; 12 Division of Hematology, Mayo Clinic, Department of Internal Medicine, Division of Hematology, Rochester, MN; 13 Department of Hematology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea, Republic of (South); 14 Janssen Research & Development, LLC, Raritan, NJ; 15 Janssen Research & Development, LLC, Spring House, PA; 16 Hôpital Saint-Louis, Université Paris Diderot, Paris, France This study was funded by Janssen Research & Development LLC and Geron Corporation

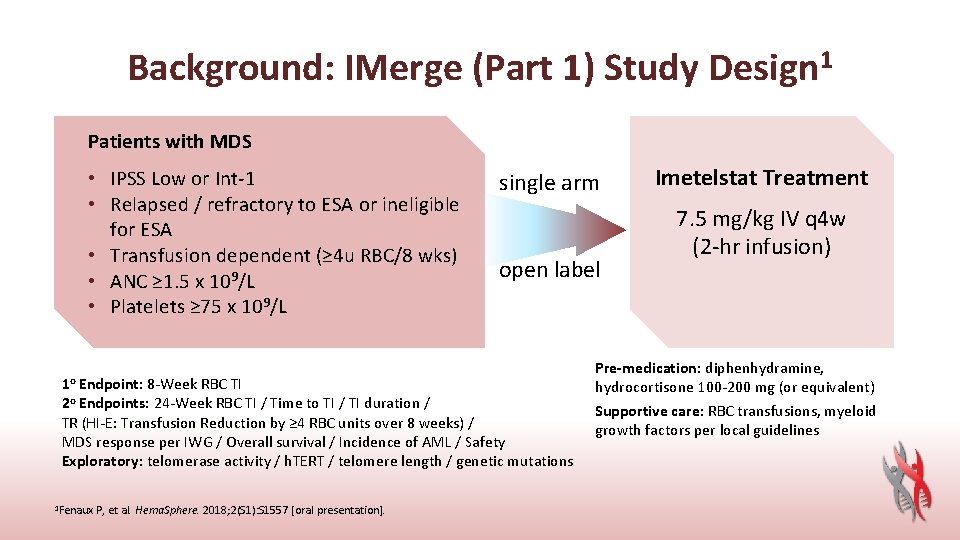
Background: IMerge (Part 1) Study Design 1 Patients with MDS • IPSS Low or Int-1 • Relapsed / refractory to ESA or ineligible for ESA • Transfusion dependent (≥ 4 u RBC/8 wks) • ANC ≥ 1. 5 x 109/L • Platelets ≥ 75 x 109/L 1 o Endpoint: 8 -Week RBC TI single arm open label 2 o Endpoints: 24 -Week RBC TI / Time to TI / TI duration / TR (HI-E: Transfusion Reduction by ≥ 4 RBC units over 8 weeks) / MDS response per IWG / Overall survival / Incidence of AML / Safety Exploratory: telomerase activity / h. TERT / telomere length / genetic mutations 1 Fenaux P, et al. Hema. Sphere. 2018; 2(S 1): S 1557 [oral presentation]. Imetelstat Treatment 7. 5 mg/kg IV q 4 w (2 -hr infusion) Pre-medication: diphenhydramine, hydrocortisone 100 -200 mg (or equivalent) Supportive care: RBC transfusions, myeloid growth factors per local guidelines

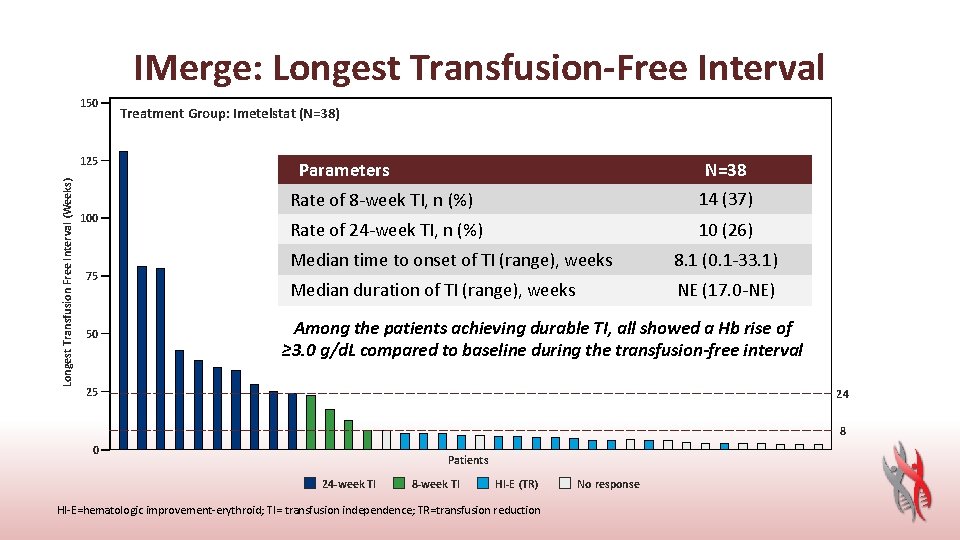
IMerge: Longest Transfusion-Free Interval 150 Longest Transfusion Free Interval (Weeks) 125 100 75 50 Treatment Group: Imetelstat (N=38) Parameters N=38 Rate of 8 -week TI, n (%) 14 (37) Rate of 24 -week TI, n (%) 10 (26) Median time to onset of TI (range), weeks 8. 1 (0. 1 -33. 1) Median duration of TI (range), weeks NE (17. 0 -NE) Among the patients achieving durable TI, all showed a Hb rise of ≥ 3. 0 g/d. L compared to baseline during the transfusion-free interval 25 24 8 0 Patients 24 -week TI 8 -week TI HI-E (TR) HI-E=hematologic improvement-erythroid; TI= transfusion independence; TR=transfusion reduction No response

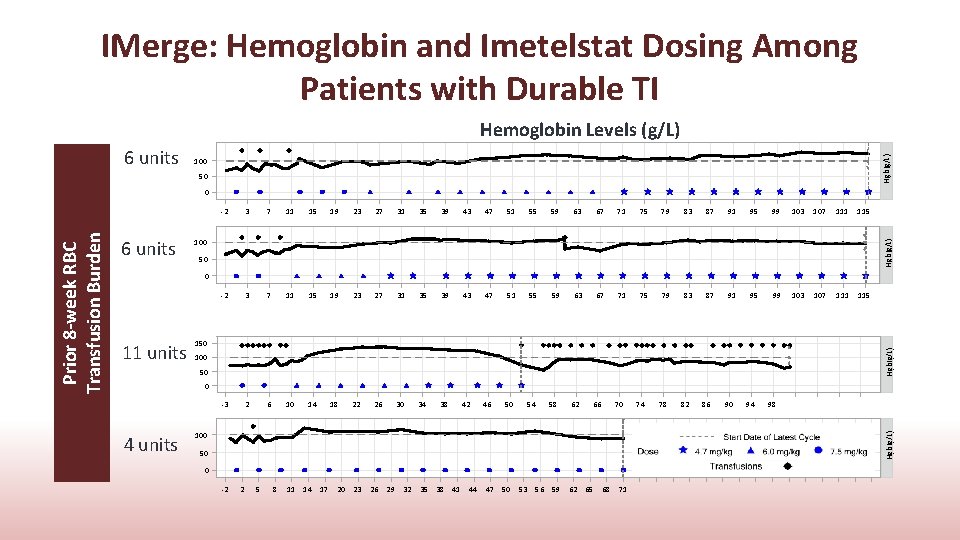
IMerge: Hemoglobin and Imetelstat Dosing Among Patients with Durable TI 6 units Hgb(g/L) Hemoglobin Levels (g/L) 100 50 3 7 11 15 19 23 27 31 35 39 43 47 51 55 59 63 67 71 75 79 83 87 91 95 99 103 107 111 115 -2 3 7 11 15 19 23 27 31 35 39 43 47 51 55 59 63 67 71 75 79 83 87 91 95 99 103 107 111 115 -3 2 6 10 14 18 22 26 30 34 38 42 46 50 54 100 Hgb(g/L) 6 units -2 50 0 150 Hgb(g/L) 11 units 100 50 0 4 units 58 62 66 70 100 74 78 82 86 90 94 98 Hgb(g/L) Prior 8 -week RBC Transfusion Burden 0 50 0 -2 2 5 8 11 14 17 20 23 26 29 32 35 38 41 44 47 50 53 56 59 62 65 68 71

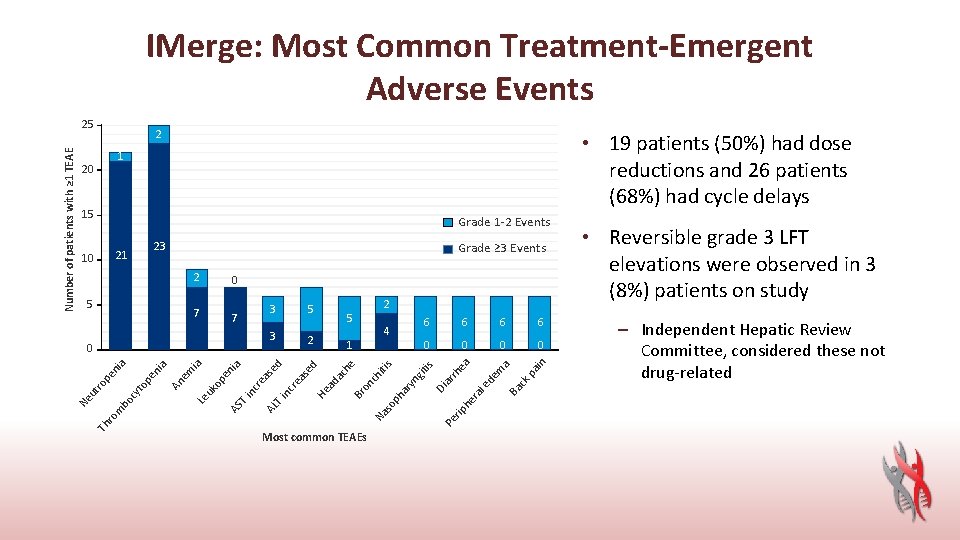
IMerge: Most Common Treatment-Emergent Adverse Events 2 • 19 patients (50%) had dose reductions and 26 patients (68%) had cycle delays 1 20 15 Grade 1 -2 Events 23 2 6 0 0 rh he ra ar yn git i ar ph l e de m a Ba ck p ain 6 ea 6 s s iti Most common TEAEs 6 Di 1 so T i nc re as nc T i AS ed ed ia en op Le uk em ia An a pe ni 2 4 Pe rip Th ro m bo cy to Ne ut ro pe ni a 0 5 ch 3 2 he 7 5 Na 3 Br on 7 ac 5 0 ad 10 Grade ≥ 3 Events He 21 AL Number of patients with ≥ 1 TEAE 25 • Reversible grade 3 LFT elevations were observed in 3 (8%) patients on study – Independent Hepatic Review Committee, considered these not drug-related

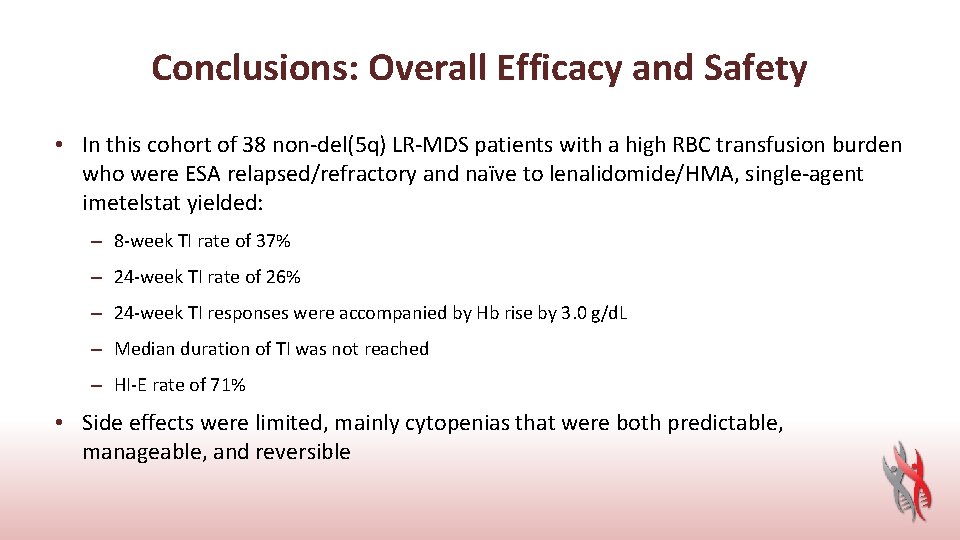
Conclusions: Overall Efficacy and Safety • In this cohort of 38 non-del(5 q) LR-MDS patients with a high RBC transfusion burden who were ESA relapsed/refractory and naïve to lenalidomide/HMA, single-agent imetelstat yielded: – 8 -week TI rate of 37% – 24 -week TI rate of 26% – 24 -week TI responses were accompanied by Hb rise by 3. 0 g/d. L – Median duration of TI was not reached – HI-E rate of 71% • Side effects were limited, mainly cytopenias that were both predictable, manageable, and reversible

A Phase II Study of Nivolumab or Ipilimumab with or without Azacitidine for Patients with Myelodysplastic Syndrome Guillermo Garcia-Manero, Koji Sasaki, Guillermo Montalban-Bravo, Naval G. Daver, Elias J. Jabbour, Yesid Alvarado, Courtney D. Di. Nardo, Farhad Ravandi, Gautam Borthakur, Prithviraj Bose, Naveen Pemmaraju, Kiran Naqvi, Jorge E. Cortes, Tapan M. Kadia, Marina Y. Konopleva, Simona Colla, Hui Yang, Caitlin R. Rausch, Yvonne Gasior, Carlos E. Bueso-Ramos, Rashmi Kanagal-Shamanna, Keyur P. Patel, Hagop M. Kantarjian Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX

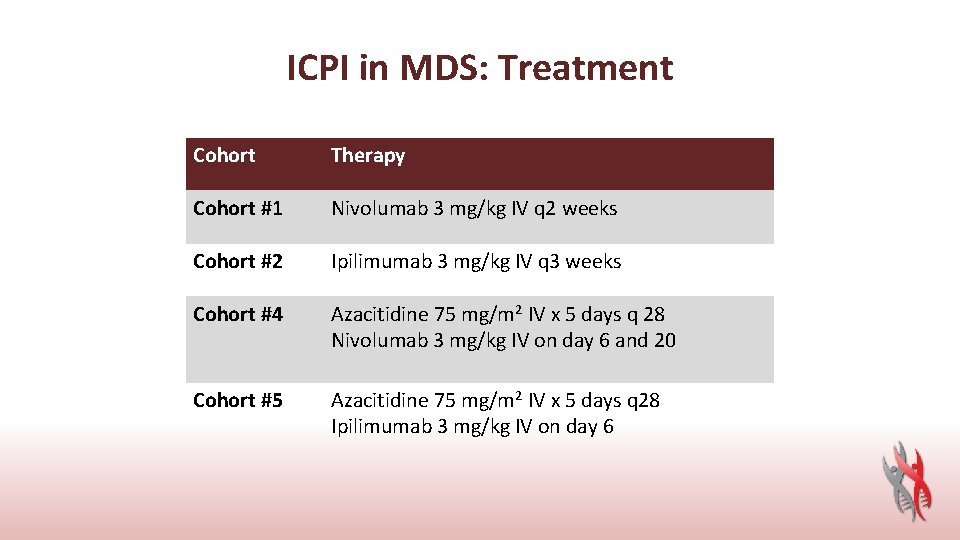
ICPI in MDS: Treatment Cohort Therapy Cohort #1 Nivolumab 3 mg/kg IV q 2 weeks Cohort #2 Ipilimumab 3 mg/kg IV q 3 weeks Cohort #4 Azacitidine 75 mg/m 2 IV x 5 days q 28 Nivolumab 3 mg/kg IV on day 6 and 20 Cohort #5 Azacitidine 75 mg/m 2 IV x 5 days q 28 Ipilimumab 3 mg/kg IV on day 6

ICPI in MDS: Patient Characteristics Frontline HMA failure Nivo + AZA N=20 Ipi + AZA N=21 Nivo N=15 Ipi N=20 Age 66 [39. 5 -83. 3] 72 [45. 1 -85. 0] 78 [61. 8 -84. 8] 69 [48. 1 -85. 7] Male 16 (80) 12 (57) 8 (53) 10 (50) PS ≥ 2 1 (5) 5 (24) 2 (13) 3 (15) WBC 5. 1 (1. 7 -48. 2) 3. 4 (0. 5 -22. 1) 1. 8 (0. 8 -16. 3) 2. 0 (0. 9 -21. 3) ANC 2. 2 (0. 0 -31. 8) 1. 9 (0. 1 -10. 6) 0. 4 (0. 1 -9. 0) 0. 7 (0. 2 -13. 4) Hgb 9. 6 (7. 3 -14. 6) 9. 8 (5. 2 -13. 3) 9. 5 (6. 5 -11. 2) 9. 1 (6. 5 -13. 8) Plt 54 (6 -224) 62 (2 -319) 31 (12 -282) 43 (2 -189) 9 (1 -18) 8 (1 -18) 4 (1 -13) 4 (0 -15) MDS 13 (65) 17 (81) 14 (93) 16 (80) MDS/MPN 6 (30) 4 (19) 1 (7) 4 (20) AML 1 (5) 0 0 0 BM blasts Dx group

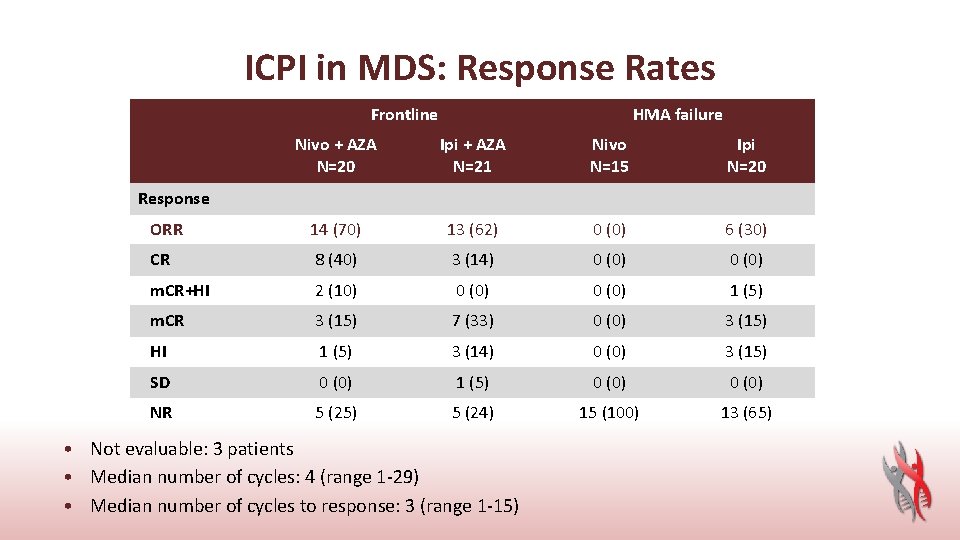
ICPI in MDS: Response Rates Frontline HMA failure Nivo + AZA N=20 Ipi + AZA N=21 Nivo N=15 Ipi N=20 ORR 14 (70) 13 (62) 0 (0) 6 (30) CR 8 (40) 3 (14) 0 (0) m. CR+HI 2 (10) 0 (0) 1 (5) m. CR 3 (15) 7 (33) 0 (0) 3 (15) HI 1 (5) 3 (14) 0 (0) 3 (15) SD 0 (0) 1 (5) 0 (0) NR 5 (25) 5 (24) 15 (100) 13 (65) Response • Not evaluable: 3 patients • Median number of cycles: 4 (range 1 -29) • Median number of cycles to response: 3 (range 1 -15)

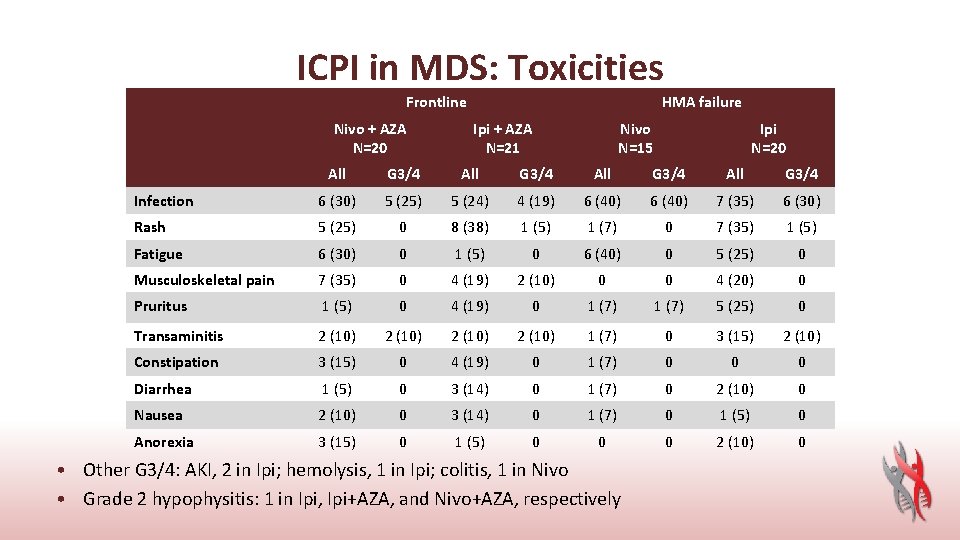
ICPI in MDS: Toxicities Frontline Nivo + AZA N=20 HMA failure Ipi + AZA N=21 Nivo N=15 Ipi N=20 All G 3/4 Infection 6 (30) 5 (25) 5 (24) 4 (19) 6 (40) 7 (35) 6 (30) Rash 5 (25) 0 8 (38) 1 (5) 1 (7) 0 7 (35) 1 (5) Fatigue 6 (30) 0 1 (5) 0 6 (40) 0 5 (25) 0 Musculoskeletal pain 7 (35) 0 4 (19) 2 (10) 0 0 4 (20) 0 Pruritus 1 (5) 0 4 (19) 0 1 (7) 5 (25) 0 Transaminitis 2 (10) 1 (7) 0 3 (15) 2 (10) Constipation 3 (15) 0 4 (19) 0 1 (7) 0 0 0 Diarrhea 1 (5) 0 3 (14) 0 1 (7) 0 2 (10) 0 Nausea 2 (10) 0 3 (14) 0 1 (7) 0 1 (5) 0 Anorexia 3 (15) 0 1 (5) 0 0 0 2 (10) 0 • Other G 3/4: AKI, 2 in Ipi; hemolysis, 1 in Ipi; colitis, 1 in Nivo • Grade 2 hypophysitis: 1 in Ipi, Ipi+AZA, and Nivo+AZA, respectively

PD-L 1 Blockade with Atezolizumab in Higher-Risk Myelodysplastic Syndrome: An Initial Safety and Efficacy Analysis Aaron T Gerds, 1 Bart L Scott, 2 Peter Greenberg, 3 Samer Khaled, 4 Tara L Lin, 5 Daniel A Pollyea, 6 Amit Verma, 7 Monique Dail, 8 Cherie Green, 8 Connie Ma, 8 Bruno C Medeiros, 8 Patrick Phuong, 8 Michael Wenger, 9 Mark Yan, 10 William Donnellan 11 1 Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA; 2 Fred Hutchinson Cancer Research Center, Seattle, WA, USA; 3 Stanford Cancer Institute, Stanford, CA, USA; 4 City of Hope, Duarte, CA, USA; 5 University of Kansas Medical Center, Kansas City, KS, USA; 6 University of Colorado School of Medicine, Aurora, CO, USA; 7 Albert Einstein College of Medicine and Montefiore Medical Center, Bronx, NY, USA; 8 Genentech, Inc. , South San Francisco, CA, USA; 9 F. Hoffmann-La Roche Ltd, Basel, Switzerland; 10 Hoffmann-La Roche Ltd, Mississauga, ON, Canada; 11 Sarah Cannon Research Institute/Tennessee Oncology, Nashville, TN, USA

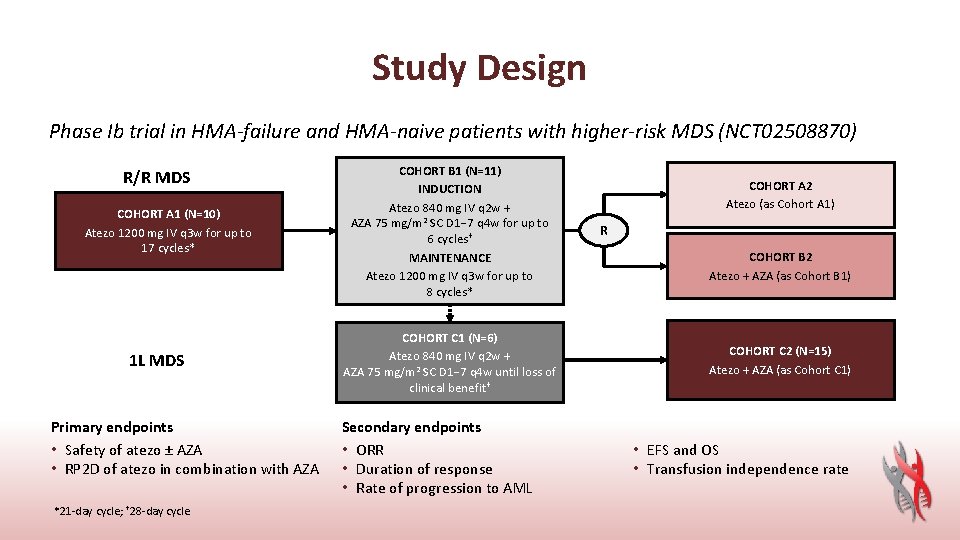
Study Design Phase Ib trial in HMA-failure and HMA-naive patients with higher-risk MDS (NCT 02508870) R/R MDS COHORT A 1 (N=10) Atezo 1200 mg IV q 3 w for up to 17 cycles* 1 L MDS Primary endpoints • Safety of atezo ± AZA • RP 2 D of atezo in combination with AZA *21 -day cycle; † 28 -day cycle COHORT B 1 (N=11) INDUCTION Atezo 840 mg IV q 2 w + AZA 75 mg/m 2 SC D 1− 7 q 4 w for up to 6 cycles† MAINTENANCE Atezo 1200 mg IV q 3 w for up to 8 cycles* COHORT C 1 (N=6) Atezo 840 mg IV q 2 w + AZA 75 mg/m 2 SC D 1− 7 q 4 w until loss of clinical benefit † Secondary endpoints • ORR • Duration of response • Rate of progression to AML COHORT A 2 Atezo (as Cohort A 1) R COHORT B 2 Atezo + AZA (as Cohort B 1) COHORT C 2 (N=15) Atezo + AZA (as Cohort C 1) • EFS and OS • Transfusion independence rate

Baseline Characteristics (N=42) Characteristic Median age, years (range) Male, n (%) Bone marrow blasts, median (range), % Cytogenetics, n (%) Good or very good Intermediate or worse IPSS-R at screening, n (%) Intermediate risk High risk Very high risk Prior lines of therapy, n (%) 1 ≥ 2 Clinical cut-off date (CCOD): 12 January 2018 Cohort A 1 Atezo (n=10) Cohort B 1 Atezo + AZA (n=11) Cohort C 1/C 2 (1 L) Atezo + AZA (n=21) 72 (63– 87) 76 (67– 89) 72 (50– 81) 8 (80) 7 (64) 15 (71) 5 (2– 14) 1 (1– 17) 5 (0– 15) 5 (50) 7 (64) 4 (36) 8 (38) 13 (62) NA NA 9 (43) 6 (29) 5 (50) 6 (55) 5 (45) NA

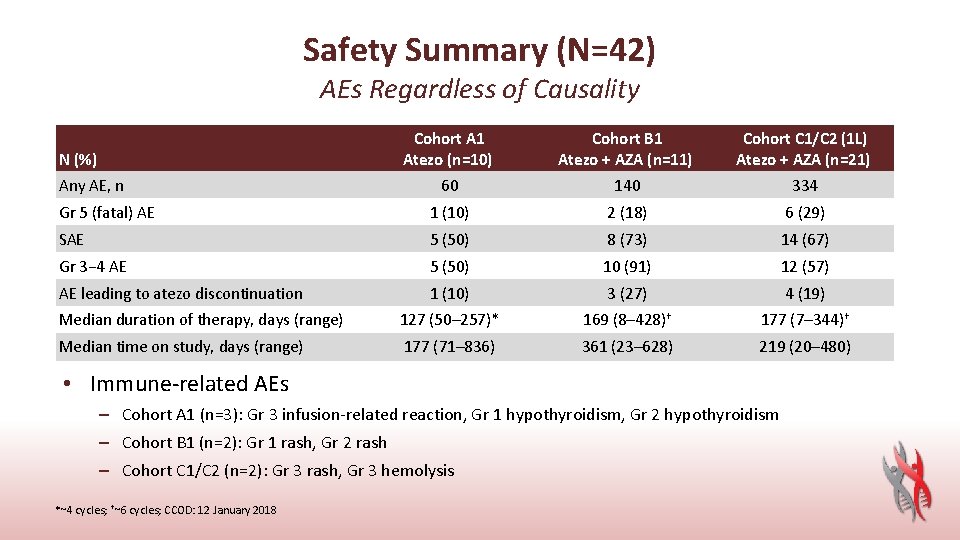
Safety Summary (N=42) AEs Regardless of Causality Cohort A 1 Atezo (n=10) Cohort B 1 Atezo + AZA (n=11) Cohort C 1/C 2 (1 L) Atezo + AZA (n=21) 60 140 334 Gr 5 (fatal) AE 1 (10) 2 (18) 6 (29) SAE 5 (50) 8 (73) 14 (67) Gr 3− 4 AE 5 (50) 10 (91) 12 (57) AE leading to atezo discontinuation 1 (10) 3 (27) 4 (19) Median duration of therapy, days (range) 127 (50– 257)* 169 (8– 428)† 177 (7– 344)† Median time on study, days (range) 177 (71– 836) 361 (23– 628) 219 (20– 480) N (%) Any AE, n • Immune-related AEs – Cohort A 1 (n=3): Gr 3 infusion-related reaction, Gr 1 hypothyroidism, Gr 2 hypothyroidism – Cohort B 1 (n=2): Gr 1 rash, Gr 2 rash – Cohort C 1/C 2 (n=2): Gr 3 rash, Gr 3 hemolysis *~4 cycles; †~6 cycles; CCOD: 12 January 2018

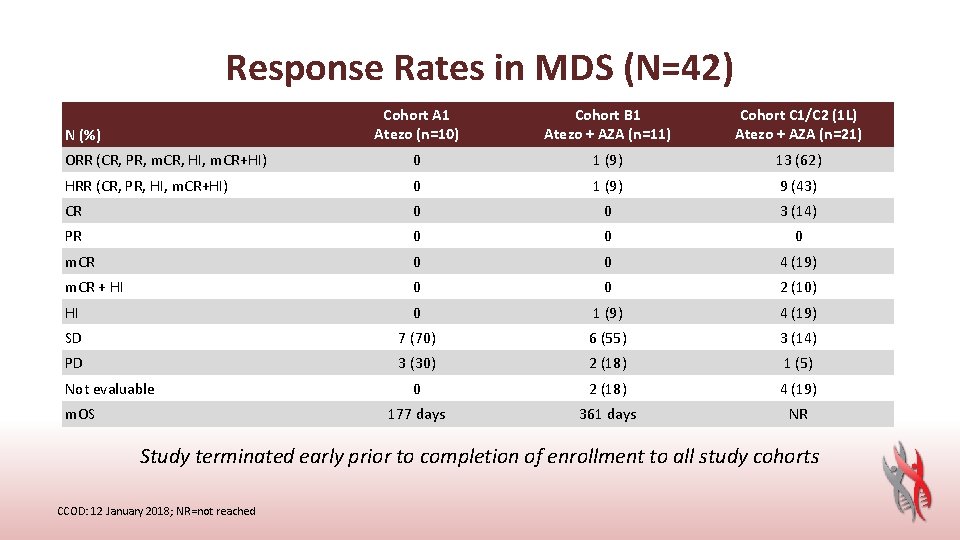
Response Rates in MDS (N=42) Cohort A 1 Atezo (n=10) Cohort B 1 Atezo + AZA (n=11) Cohort C 1/C 2 (1 L) Atezo + AZA (n=21) ORR (CR, PR, m. CR, HI, m. CR+HI) 0 1 (9) 13 (62) HRR (CR, PR, HI, m. CR+HI) 0 1 (9) 9 (43) CR 0 0 3 (14) PR 0 0 0 m. CR 0 0 4 (19) m. CR + HI 0 0 2 (10) HI 0 1 (9) 4 (19) SD 7 (70) 6 (55) 3 (14) PD 3 (30) 2 (18) 1 (5) 0 2 (18) 4 (19) 177 days 361 days NR N (%) Not evaluable m. OS Study terminated early prior to completion of enrollment to all study cohorts CCOD: 12 January 2018; NR=not reached

Phase 1 b/2 Combination Study of APR-246 and Azacitidine (AZA) in Patients with TP 53 Mutant Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML) 3, Kendra Sweet 1, Thomas Cluzeau 4, Mikkael David A Sallman 1, Amy De. Zern 2, David P Steensma Sekkeres 5, Guillermo Garcia-Manero 6, Gail Roboz 7, Amy Mc. Lemore 1, Kathy Mc. Graw 1, John Puskas 1, Ling Zhang 1, Chirag Bhagat 8, Jiqiang Yao 9, Najla H Al Ali 1, Eric Padron 1, Roger Tell 10, Jeffrey E. Lancet 1, Pierre Fenaux 11, Alan F List 1 and Rami S Komrokji 1 1 Malignant Hematology Department, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA. ; 2 Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA; 3 Department of Medical Oncology, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA; 4 Cote D’azur University, Nice Sophia Antipolis University, Hematology Department, CHU Nice, France; 5 Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland, OH, USA; 6 Department of Leukemia, MD Anderson Cancer Center, Houston, TX, USA; 7 Weill Cornell Medical College, New York, NY, USA; 9 Cancer Informatics Core, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA; 10 Aprea Therapeutics, Stockholm, Sweden; 11 Hospital St Louis, Paris 7 University, Paris, France. 2018 ASH Abstract #3091

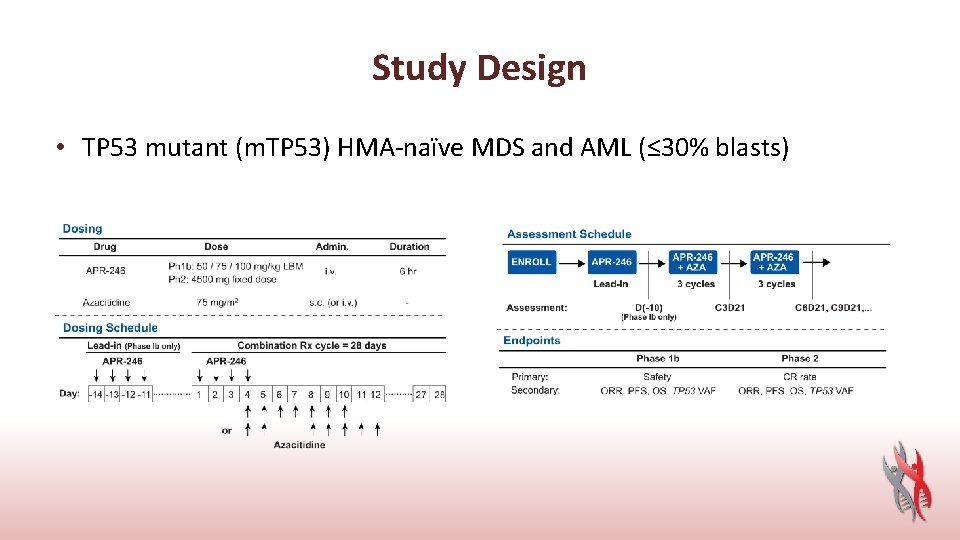
Study Design • TP 53 mutant (m. TP 53) HMA-naïve MDS and AML (≤ 30% blasts)

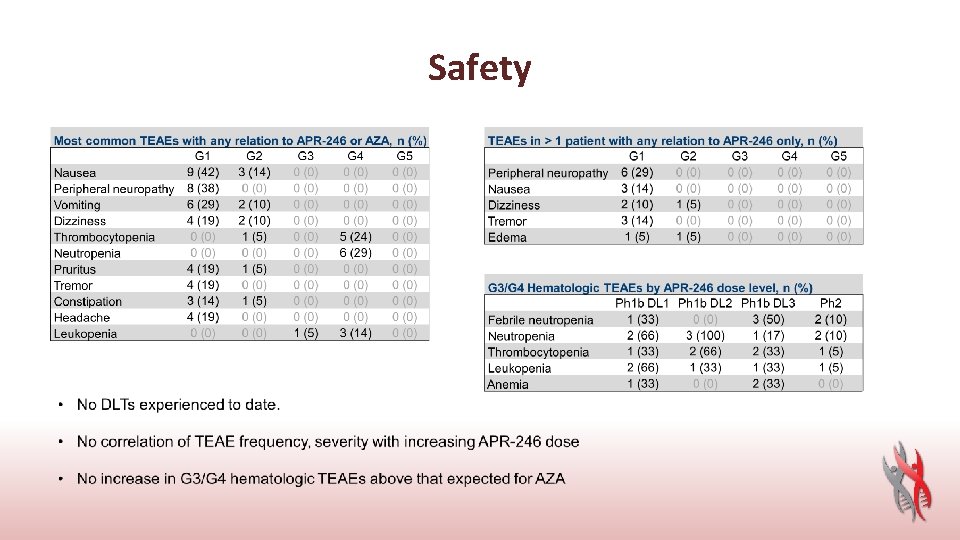
Safety

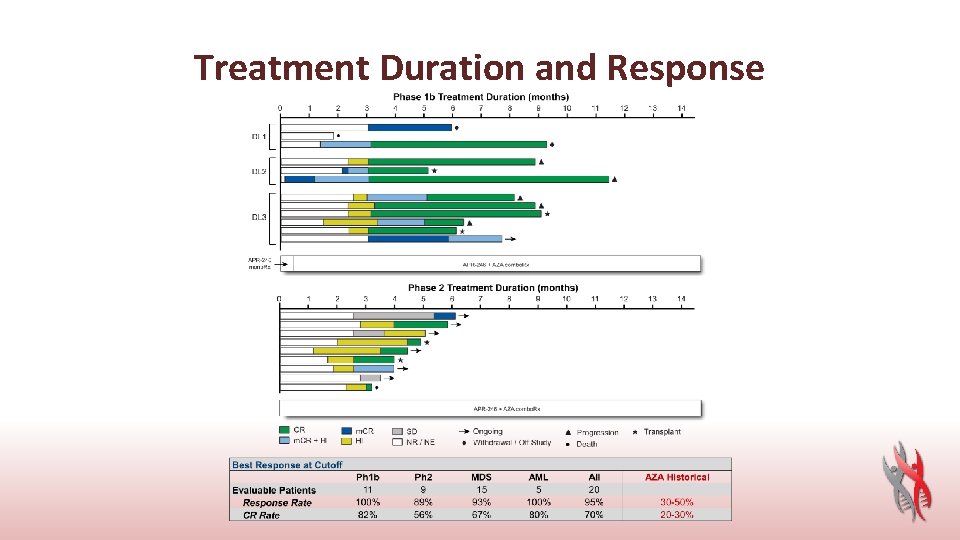
Treatment Duration and Response

High-risk MDS: New Strategies Current and Ongoing Trials • SGI-110 guadecitabine a next-generation oral HMA • Pracinostat (HDACi) in combination with azacitidine (phase 2 interim analysis) • Oral rigosertib in combination with azacitidine (phase 2 expansion study) • ASTX 727 a unique fixed-dose combination of the hypomethylating agent decitabine, and the novel cytidine deaminase inhibitor, E 7727 (cedazuridine) (phase 3) • Venetoclax
 X.next = x.next.next
X.next = x.next.next The next treatment
The next treatment Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Fecboak
Fecboak Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Dot
Dot Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi New york pennsylvania new jersey delaware
New york pennsylvania new jersey delaware Fresh oil new wine
Fresh oil new wine Strengths of the articles of confederation
Strengths of the articles of confederation Marketing management
Marketing management New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics Chapter 16 toward a new heaven and a new earth
Chapter 16 toward a new heaven and a new earth Neil thisse is a loyalist answers
Neil thisse is a loyalist answers New classical and new keynesian macroeconomics
New classical and new keynesian macroeconomics Punctuating split speech
Punctuating split speech Orchard 14 new hartford
Orchard 14 new hartford New-old approach to creating new ventures
New-old approach to creating new ventures New years old is new again
New years old is new again Roosevelt taft and wilson venn diagram
Roosevelt taft and wilson venn diagram Language windows 10
Language windows 10 Trauma awareness and treatment center utah
Trauma awareness and treatment center utah