Whats inside Properties Periodic Table of Subatomic particles

What’s inside? Properties Periodic Table # of Subatomic particles Atomic Mass - calculate
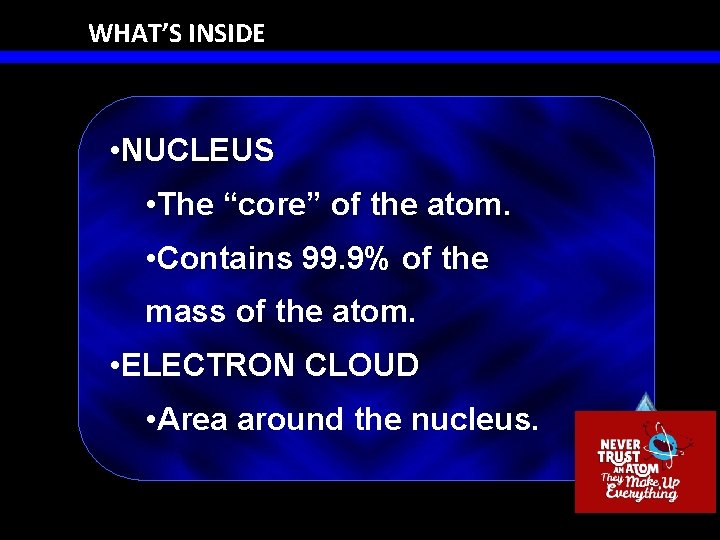
WHAT’S INSIDE • NUCLEUS • The “core” of the atom. • Contains 99. 9% of the mass of the atom. • ELECTRON CLOUD • Area around the nucleus.
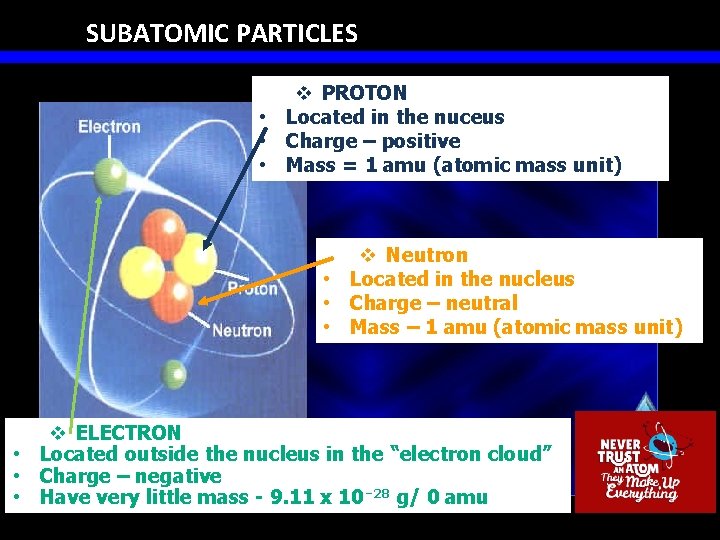
SUBATOMIC PARTICLES v PROTON • Located in the nuceus • Charge – positive • Mass = 1 amu (atomic mass unit) v Neutron • Located in the nucleus • Charge – neutral • Mass – 1 amu (atomic mass unit) v ELECTRON • Located outside the nucleus in the “electron cloud” • Charge – negative • Have very little mass - 9. 11 x 10⁻ 28 g/ 0 amu
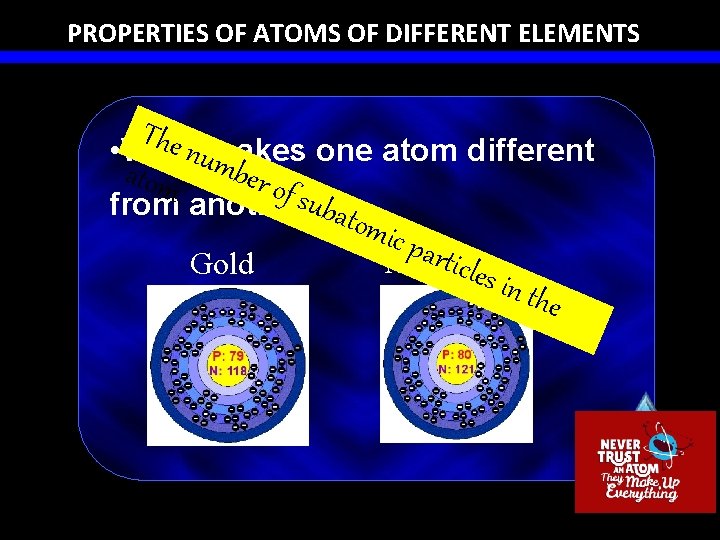
PROPERTIES OF ATOMS OF DIFFERENT ELEMENTS The • Whatnumakes one atom different m ber o atom f sub from. another? atom ic pa rticle Mercury Gold s in t he
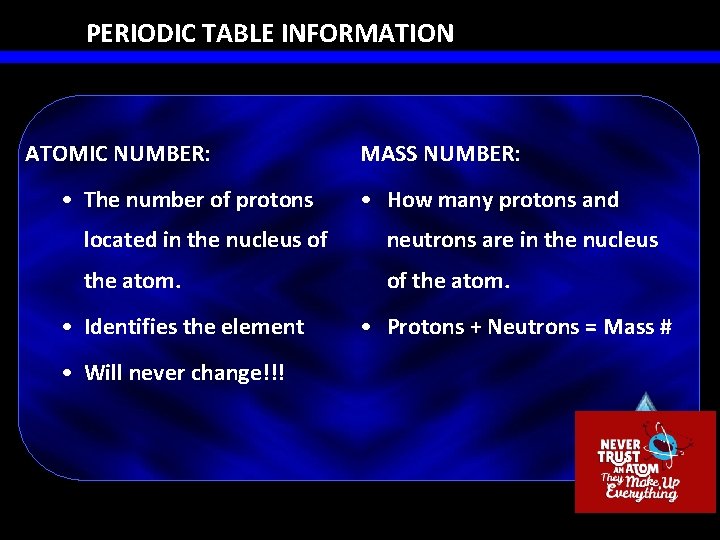
PERIODIC TABLE INFORMATION ATOMIC NUMBER: • The number of protons MASS NUMBER: • How many protons and located in the nucleus of neutrons are in the nucleus the atom. of the atom. • Identifies the element • Will never change!!! • Protons + Neutrons = Mass #

DETERMINING NUMBER OF SUBATOMIC PARTICLES Element Manganese Sodium Bromine Yttrium Number of Protons Number of Electrons Number of Neutrons Atomic Number Protons: Same as the atomic #. 25 30 Neutrons : 12 Mass 11# - Atomic # 35 45 Electrons: Same as the # of protons 39 Mass Number 89 Arsenic 33 75 Actinium 227

AVERAGE ATOMIC MASS • Number that shows on the periodic table. • Average of all the isotopes of an element. An isotope is an atom that has the same number of protons but differing numbers of neutrons, changing the mass of the atom.
CALCULATING ATOMIC MASS • Determine the mass contribution of each isotope of the element. • (relative abundance x mass of isotope) • Add the mass contributions together. • Note that you do not divide!! • Use significant figures in rounding answer.
- Slides: 8