Whats Going On with SQ 109 LMU Infectex











- Slides: 15

What‘s Going On with SQ 109 ? LMU Infectex

TB Alliance Open Forum 2 London, December 2006 “… each drug should be developed according to the specific characteristics of the drug itself, not form-fitted into a one-size-fits-all clinical development program…” [Nacy]

Timecourse for SQ 109 Effects In Vivo Drug Regimen Log 10 CFU in Lung Log Decrease Untreated 6. 16 ± 0. 02 INH+RIF+EMB 4. 64 ± 0. 23 INH+RIF+SQ 109 4. 46 ± 0. 12 1. 52 1. 70 2 Weeks 3 Weeks Untreated 6. 34 ± 0. 34 INH+RIF+EMB 4. 38± 0. 05 INH+RIF+SQ 109 3. 80 ± 0. 10 1. 96 2. 54 4 Weeks Untreated 6. 42 ± 0. 76 INH+RIF+EMB 3. 86 ± 0. 14 INH+RIF+SQ 109 3. 26 ± 0. 12 2. 56 3. 16 SQ 109 10 mg/kg; INH 25 mg/kg; RIF 20 mg/kg; EMB 100 mg/kg Nikonenko, et al. 2007. Drug therapy of experimental tuberculosis (TB): improved outcome by combining SQ 109, a new diamine antibiotic, with existing TB drugs. Antimicrob. Agents and Chemother 51: 1553.

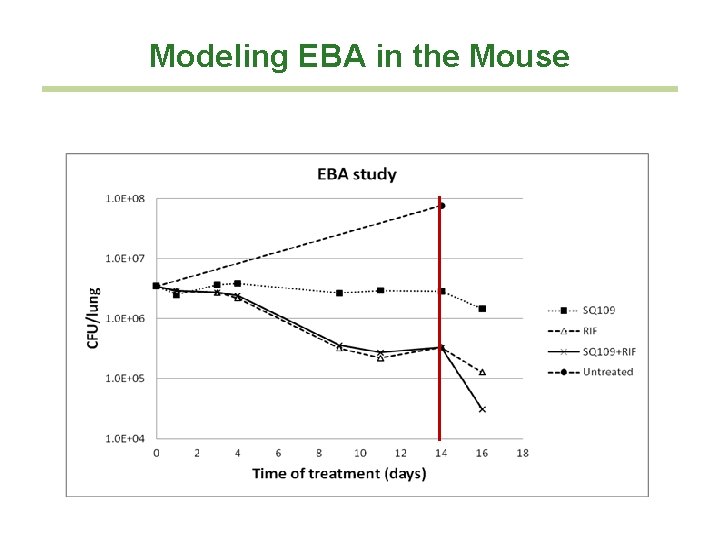
Modeling EBA in the Mouse

Timecourse for SQ 109 Effects In Vivo Drug Log 10 CFU in Lung Log Increase or Decrease From Day 0 Control Log Increase or Decrease From Day 30 Control Day 0 Day 30 No Treatment 6. 54 ± 0. 40 8. 18 ± 0. 11 +1. 64 0 SQ 109 6. 54 ± 0. 40 6. 94 ± 0. 20 +0. 40 -1. 24 RIF 6. 54 ± 0. 40 3. 64 ± 0. 15 -2. 90 -4. 54 SQ 109+RIF 6. 54 ± 0. 40 3. 34 ± 0. 03 -3. 20 -4. 84 Experiment: EBA SQ 109 10 mg/kg; RIF 20 mg/kg; SQ 109 reduced replication of Mtb and improved RIF activity (1/3 log 10 CFU) by day 30

TB Alliance Open Forum 2 London, December 2006 “… each drug should be developed according to the specific characteristics of the drug itself, not form-fitted into a one-size-fits-all clinical development program…” [Nacy] [TMC 207 shows minimal activity in EBA] “…unfortunately, a right-of-passage in the TB community…” [Tibotec]

Individual Patient Log 10 CFU over Time

EBA with 95% confidence intervals

Mixed Effects Model Assuming Linear Decline Fitted estimates of difference from mean baseline log 10(CFU) /ml by visit and treatment allocation

Individual Patient Log 10 CFU over Time

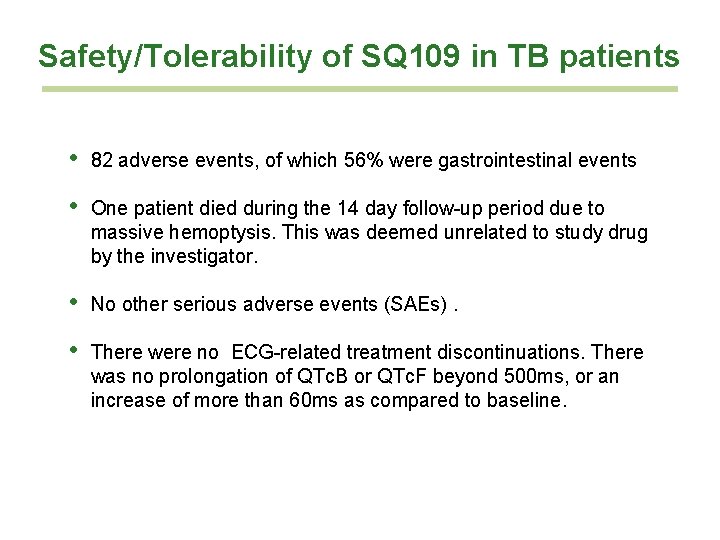
Safety/Tolerability of SQ 109 in TB patients • 82 adverse events, of which 56% were gastrointestinal events • One patient died during the 14 day follow-up period due to massive hemoptysis. This was deemed unrelated to study drug by the investigator. • No other serious adverse events (SAEs). • There were no ECG-related treatment discontinuations. There was no prolongation of QTc. B or QTc. F beyond 500 ms, or an increase of more than 60 ms as compared to baseline.

EBA Conclusions SQ 109 is a safe and well tolerated drug. It‘s main side effect is nausea, which is more pronounced in the 300 mg dose There were no systematic increases in QT in the SQ 109 groups Steady state appears to be reached at ~day 7; the induction of CYP 2 C 19 through Rif can be overcome with 300 mg SQ 109 had no bactericidal effect in humans over 14 days; RIF had a 1 -log effect in humans over 14 days. Mouse modeling data suggest that: - EBA data in humans mimics that seen in mouse - SQ 109 effects are apparent the longer the drug is taken

EBA Study Team Acknowledgments Sponsor: Chief Investigator: PI: Co-PI: Microbiology: Sponsor Medical Expert: Trial Statistician (MRC): Chief Medical Officer Sequella Inc. : Medical Center of the University of Munich Michael Hoelscher Andreas Diacon Rodney Dawson Andeas Diacon, Amour Venter Norbert Heinrich Patrick Phillips Gary Horwith Pan. ACEA Chief Investigators Group: M. Boeree, S. Gillespie, M. Hoelscher Funding: EDCTP, BMGF, BMBF, UK-MRC, Sequella, NIH

EBA Value-Add in TB Drug Development? Drugs effective in TB treatments that work poorly (or not at all) in EBA: • • • Rifampicin Linezolid Clofazimine Pyrazinamide Bedaquiline SQ 109

What‘s Next for SQ 109 • Registration trial in Russia for MDR-TB: ‒ OBT ± SQ 109 (300 mg) ‒ ICH guidelines ‒ Start: Q 4 2012 • MAMS study in Africa in DS-TB: ‒ SQ 109 (300 mg) vs EMB in SOC high-dose RIF ‒ Start: Q 4 2012 • Thorough QT (TQT) in healthy humans ‒ SQ 109 (up to 450 mg) ± moxifloxicin • New Drug Combinations in MDR-TB, ACTG • New Drug Combinations in DS-TB, ACTG Infectex
 Tic tac toe going high going low going criss cross lollipop
Tic tac toe going high going low going criss cross lollipop If you are going through hell, keep going means
If you are going through hell, keep going means Lmu lst
Lmu lst Betriebsarzt lmu goethestraße
Betriebsarzt lmu goethestraße Phonetik lmu
Phonetik lmu Grid and cloud computing lmu
Grid and cloud computing lmu Fachschaft geographie lmu
Fachschaft geographie lmu Cis lmu
Cis lmu Lmu otd
Lmu otd Kontaktstelle mathematik lmu
Kontaktstelle mathematik lmu Algorithmen und datenstrukturen lmu
Algorithmen und datenstrukturen lmu Lmu medieninformatik
Lmu medieninformatik Lmu
Lmu Fachschaft schulpsychologie lmu
Fachschaft schulpsychologie lmu Pags lmu
Pags lmu Datenbanksysteme lmu
Datenbanksysteme lmu