What You Need to Know About Percutaneous TAVI

What You Need to Know About Percutaneous TAVI Eberhard Grube MD Instituto Dante Pazzanese de Cardiologia, São Paulo, Brazil St. Elisabeth Hospital, Heart Center Rhein-Ruhr, Essen, Germany

DISCLOSURES Eberhard Grube, MD Consulting Fees – Abbott Vascular, Boston Scientific Corporation, Cordis, a Johnson & Johnson Company, Medtronic Cardio. Vascular, Inc. Honoraria – Biosensors International , Boston Scientific Corporation, Medtronic Cardio. Vascular, Inc Ownership Interest (Stocks, Stock Options or Other Ownership Interest) – Biosensors International , Medtronic Cardio. Vascular, Inc. I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference off-label use of stents and valve prosthesis.
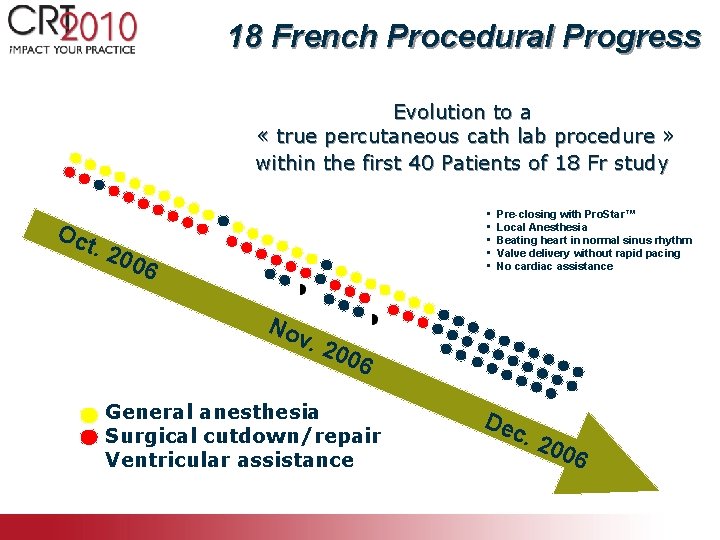
18 French Procedural Progress Evolution to a « true percutaneous cath lab procedure » within the first 40 Patients of 18 Fr study • • • Oc t. 2 006 No Pre-closing with Pro. Star™ Local Anesthesia Beating heart in normal sinus rhythm Valve delivery without rapid pacing No cardiac assistance v. 2 006 General anesthesia Surgical cutdown/repair Ventricular assistance Dec . 20 06
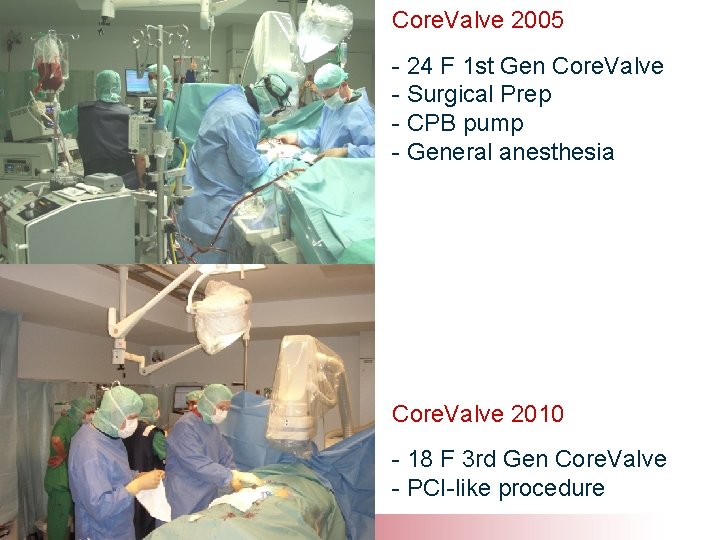
Core. Valve 2005 - 24 F 1 st Gen Core. Valve - Surgical Prep - CPB pump - General anesthesia Core. Valve 2010 - 18 F 3 rd Gen Core. Valve - PCI-like procedure

Transcatheter AVR Current Generation Devices Edwards ~7, 500 patients Core. Valve ~7, 500 patients
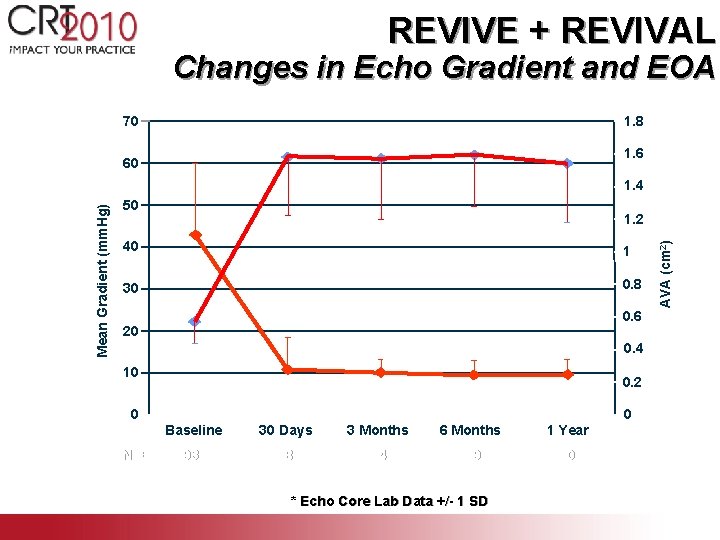
REVIVE + REVIVAL Changes in Echo Gradient and EOA * p < 0. 001 vs. baseline 70 * 60 * * * 1. 8 1. 6 50 1. 2 40 1 30 0. 8 * 20 10 0 N= 0. 6 * * * 0. 4 0. 2 0 Baseline 30 Days 3 Months 6 Months 1 Year 93 98 94 79 60 * Echo Core Lab Data +/- 1 SD AVA (cm 2) Mean Gradient (mm. Hg) 1. 4

Pre- and Post-operative Gradients Peak Gradient (mm. Hg) Mean Gradient (mm. Hg) 76. 3 75. 5 49. 6 46. 4 17. 7 17. 0 9. 3 8. 4 6
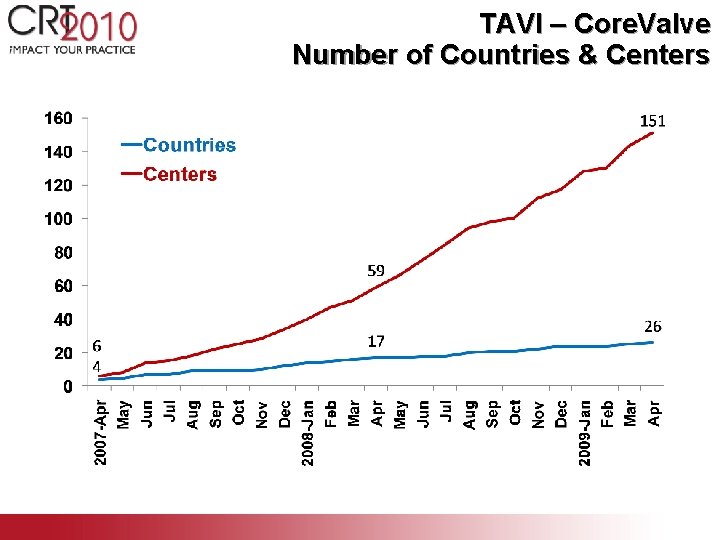
TAVI – Core. Valve Number of Countries & Centers
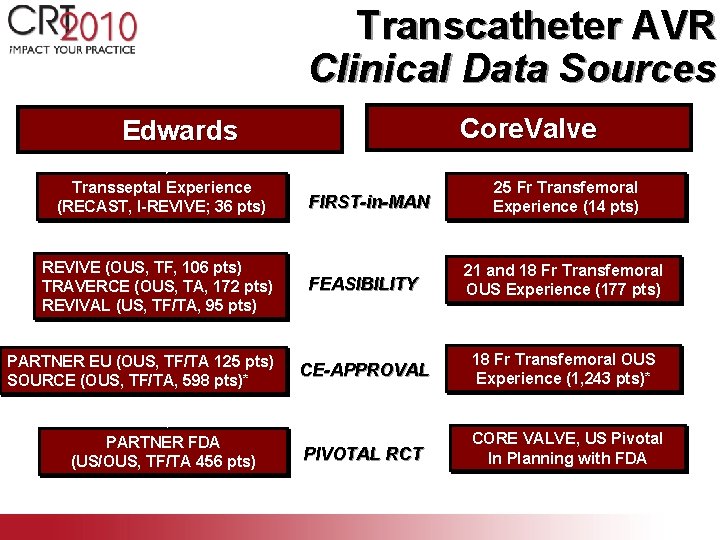
Transcatheter AVR Clinical Data Sources Core. Valve Edwards Transseptal Experience (RECAST, I-REVIVE; 36 pts) REVIVE (OUS, TF, 106 pts) TRAVERCE (OUS, TA, 172 pts) REVIVAL (US, TF/TA, 95 pts) PARTNER EU (OUS, TF/TA 125 pts) SOURCE (OUS, TF/TA, 598 pts)* PARTNER FDA (US/OUS, TF/TA 456 pts) FIRST-in-MAN 25 Fr Transfemoral Experience (14 pts) FEASIBILITY 21 and 18 Fr Transfemoral OUS Experience (177 pts) CE-APPROVAL 18 Fr Transfemoral OUS Experience (1, 243 pts)* PIVOTAL RCT CORE VALVE, US Pivotal In Planning with FDA
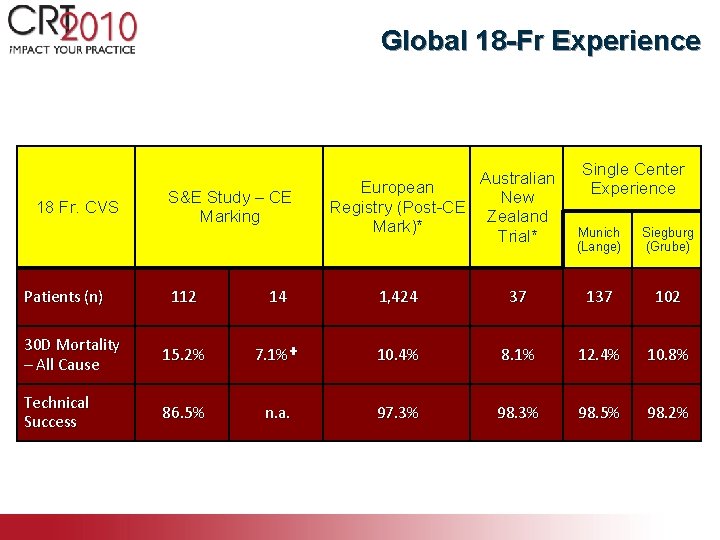
Global 18 -Fr Experience 18 Fr. CVS S&E Study – CE Marking Patients (n) Australian European New Registry (Post-CE Zealand Mark)* Trial* Single Center Experience Munich (Lange) Siegburg (Grube) 112 14 1, 424 37 102 30 D Mortality – All Cause 15. 2% 7. 1%✚ 10. 4% 8. 1% 12. 4% 10. 8% Technical Success 86. 5% n. a. 97. 3% 98. 5% 98. 2% * Site reported ✚ Un-adjudicated
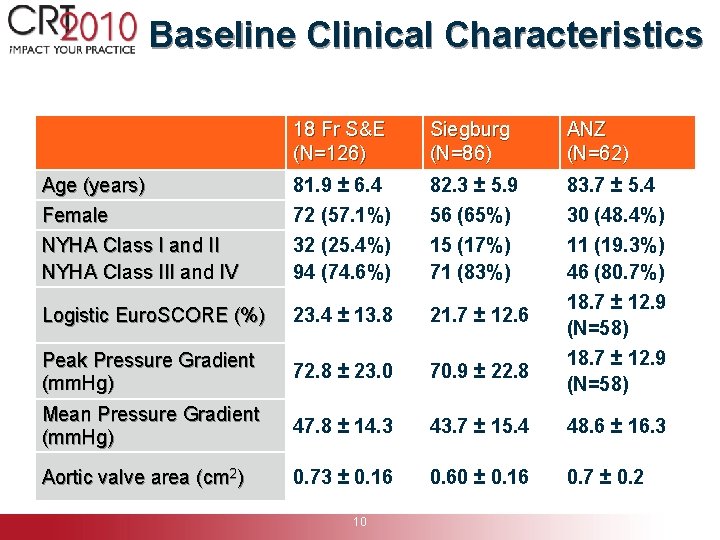
Baseline Clinical Characteristics 18 Fr S&E (N=126) Siegburg (N=86) ANZ (N=62) Age (years) Female NYHA Class I and II NYHA Class III and IV 81. 9 ± 6. 4 72 (57. 1%) 32 (25. 4%) 94 (74. 6%) 82. 3 ± 5. 9 56 (65%) 15 (17%) 71 (83%) 83. 7 ± 5. 4 30 (48. 4%) 11 (19. 3%) 46 (80. 7%) Logistic Euro. SCORE (%) 23. 4 ± 13. 8 21. 7 ± 12. 6 Peak Pressure Gradient (mm. Hg) 72. 8 ± 23. 0 70. 9 ± 22. 8 Mean Pressure Gradient (mm. Hg) 47. 8 ± 14. 3 43. 7 ± 15. 4 48. 6 ± 16. 3 Aortic valve area (cm 2) 0. 73 ± 0. 16 0. 60 ± 0. 16 0. 7 ± 0. 2 10 18. 7 ± 12. 9 (N=58)
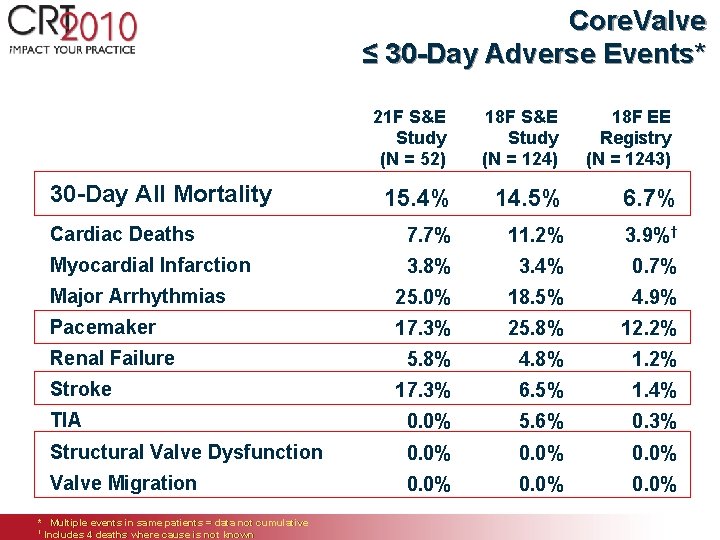
Core. Valve ≤ 30 -Day Adverse Events* 21 F S&E Study (N = 52) 30 -Day All Mortality 18 F S&E Study (N = 124) 18 F EE Registry (N = 1243) 15. 4% 14. 5% 6. 7% Cardiac Deaths 7. 7% 11. 2% 3. 9%† Myocardial Infarction 3. 8% 3. 4% 0. 7% Major Arrhythmias 25. 0% 18. 5% 4. 9% Pacemaker 17. 3% 25. 8% 12. 2% 5. 8% 4. 8% 1. 2% 17. 3% 6. 5% 1. 4% TIA 0. 0% 5. 6% 0. 3% Structural Valve Dysfunction 0. 0% Valve Migration 0. 0% Renal Failure Stroke * Multiple events in same patients = data not cumulative † Includes 4 deaths where cause is not known
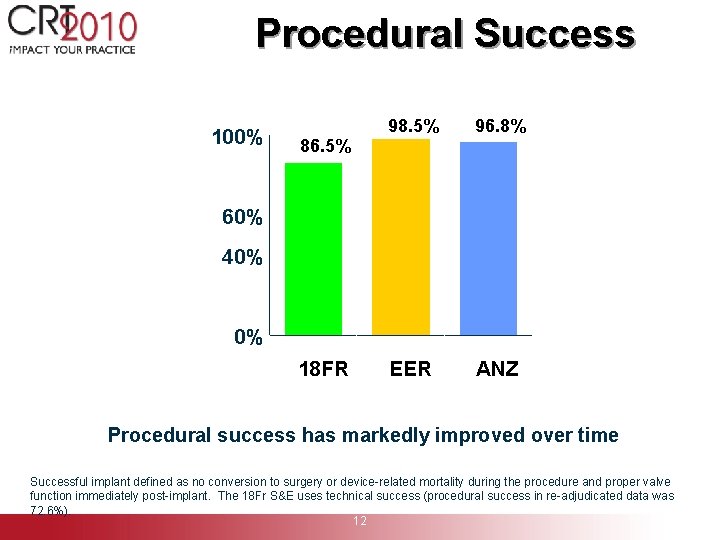
Procedural Success 100% 86. 5% 98. 5% 96. 8% EER ANZ 80% 60% 40% 20% 0% 18 FR S&E Procedural success has markedly improved over time Successful implant defined as no conversion to surgery or device-related mortality during the procedure and proper valve function immediately post-implant. The 18 Fr S&E uses technical success (procedural success in re-adjudicated data was 72. 6%). 12
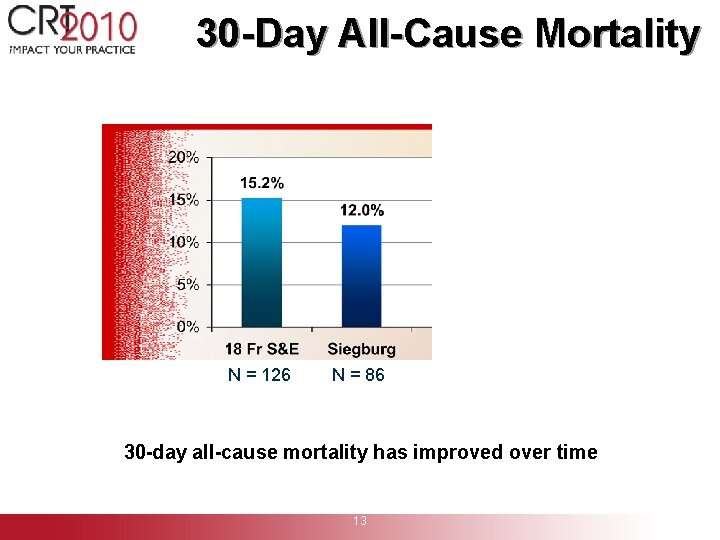
30 -Day All-Cause Mortality N = 126 N = 86 30 -day all-cause mortality has improved over time 13
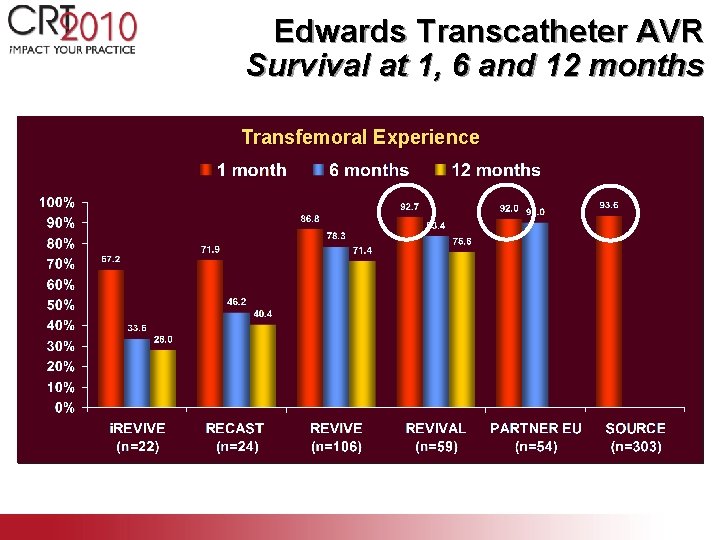
Edwards Transcatheter AVR Survival at 1, 6 and 12 months Transfemoral Experience
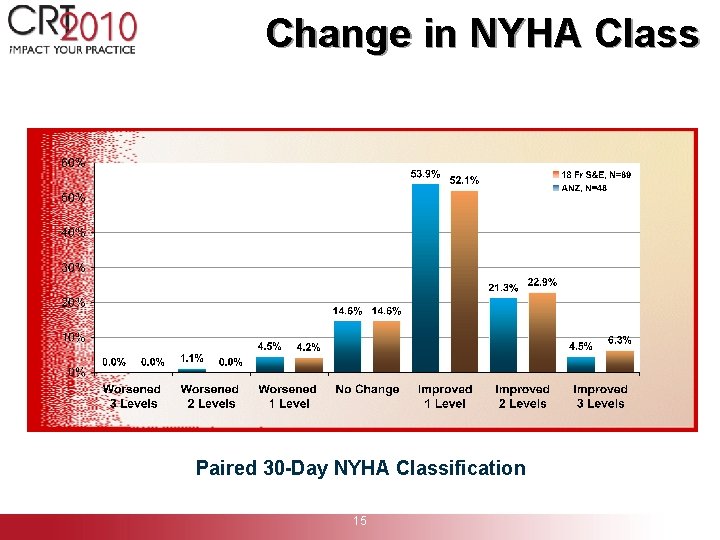
Change in NYHA Class Paired 30 -Day NYHA Classification 15
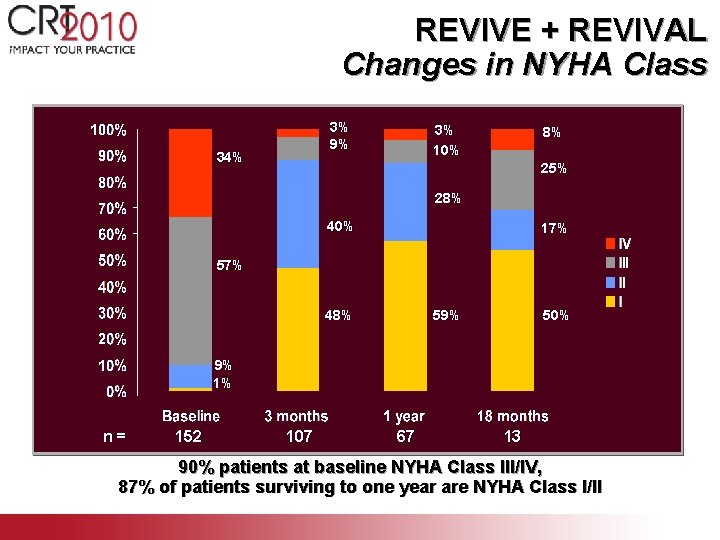
REVIVE + REVIVAL Changes in NYHA Class 3% 9% 34% 3% 10% 8% 25% 28% 40% 17% 59% 48% 50% 9% 1% n= 152 107 67 13 90% patients at baseline NYHA Class III/IV, 87% of patients surviving to one year are NYHA Class I/II
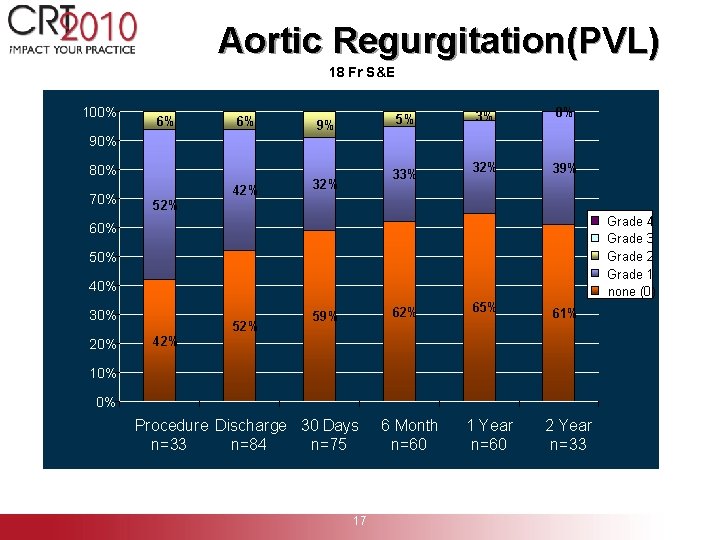
Aortic Regurgitation(PVL) 18 Fr S&E 100% 6% 6% 5% 9% 3% 0% 32% 39% 90% 80% 70% 52% 42% 33% 32% Grade 4 Grade 3 Grade 2 Grade 1 none (0) 60% 50% 40% 30% 20% 42% 52% 62% 59% 65% 61% 1 Year n=60 2 Year n=33 10% 0% Procedure Discharge 30 Days n=33 n=84 n=75 6 Month n=60 Source: 18 Fr S&E 17
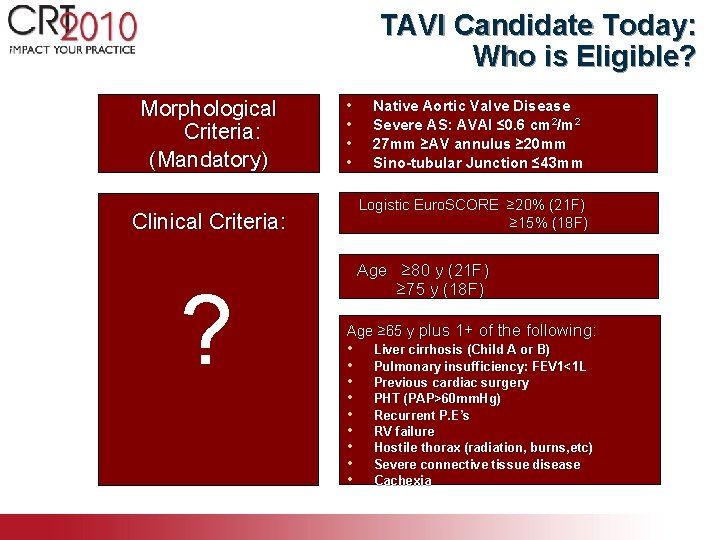
TAVI Candidate Today: Who is Eligible? Morphological Criteria: (Mandatory) • • Logistic Euro. SCORE ≥ 20% (21 F) ≥ 15% (18 F) Clinical Criteria: ? Native Aortic Valve Disease Severe AS: AVAI ≤ 0. 6 cm 2/m 2 27 mm ≥AV annulus ≥ 20 mm Sino-tubular Junction ≤ 43 mm Age ≥ 80 y (21 F) ≥ 75 y (18 F) Age ≥ 65 y plus 1+ of the following: • • • Liver cirrhosis (Child A or B) Pulmonary insufficiency: FEV 1<1 L Previous cardiac surgery PHT (PAP>60 mm. Hg) Recurrent P. E’s RV failure Hostile thorax (radiation, burns, etc) Severe connective tissue disease Cachexia
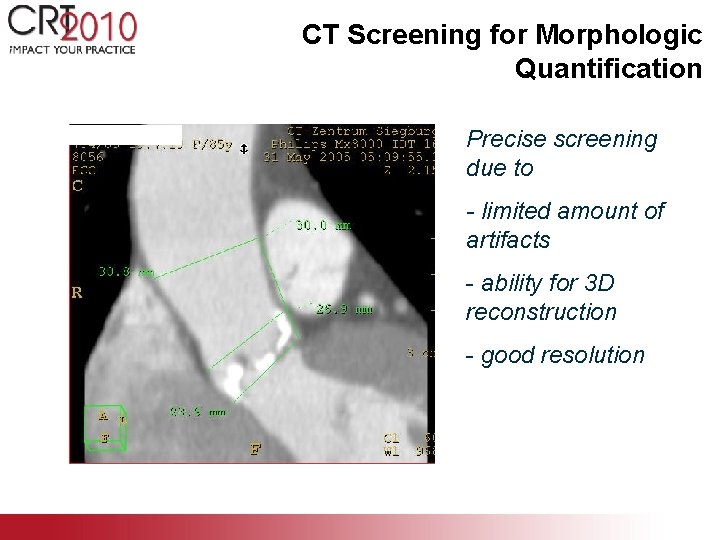
CT Screening for Morphologic Quantification Precise screening due to - limited amount of artifacts - ability for 3 D reconstruction - good resolution
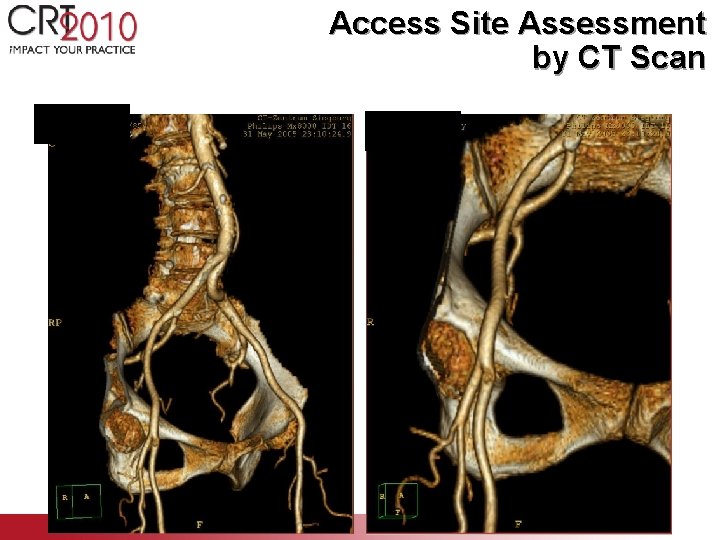
Access Site Assessment by CT Scan
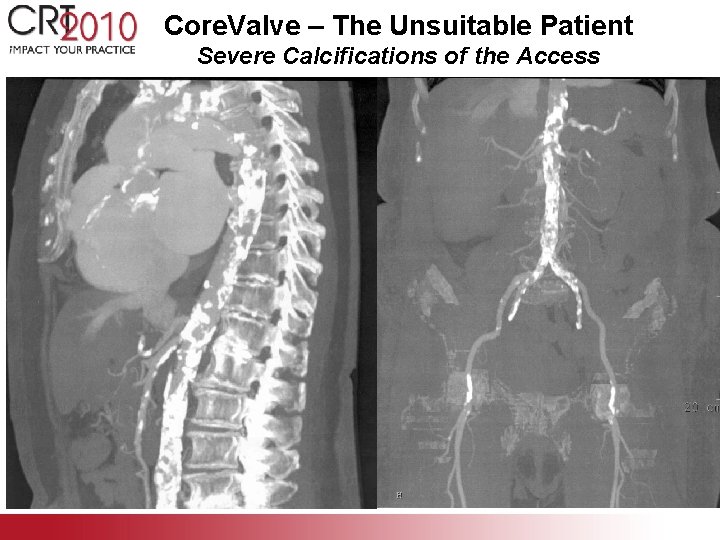
Core. Valve – The Unsuitable Patient Severe Calcifications of the Access
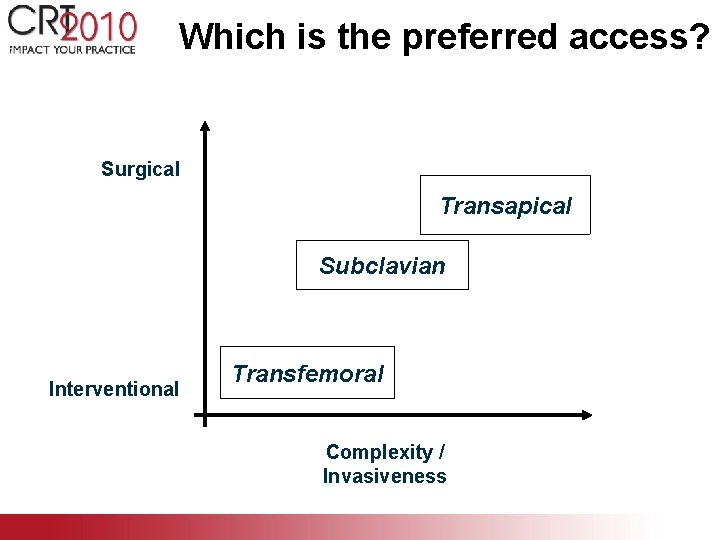
Which is the preferred access? Surgical Transapical Subclavian Interventional Transfemoral Complexity / Invasiveness
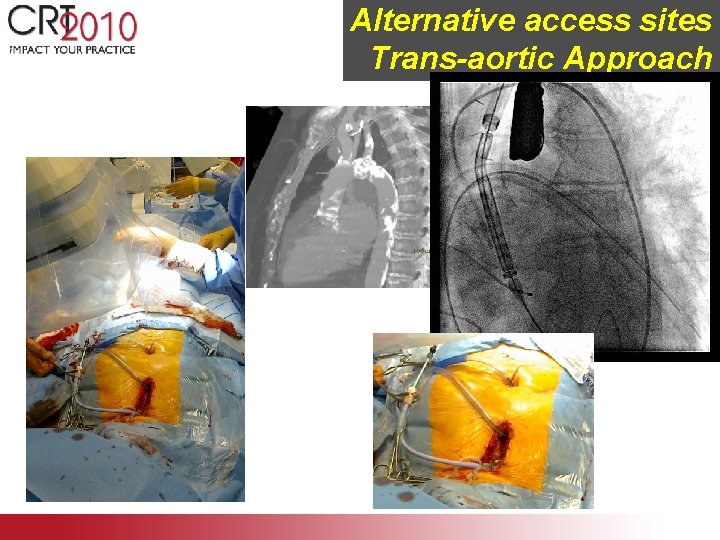
Alternative access sites Trans-aortic Approach
Future Challenges Design Features (e. g. Profile) Indication Controversies: Which Technique? Which Access Site? Which performing Discipline?
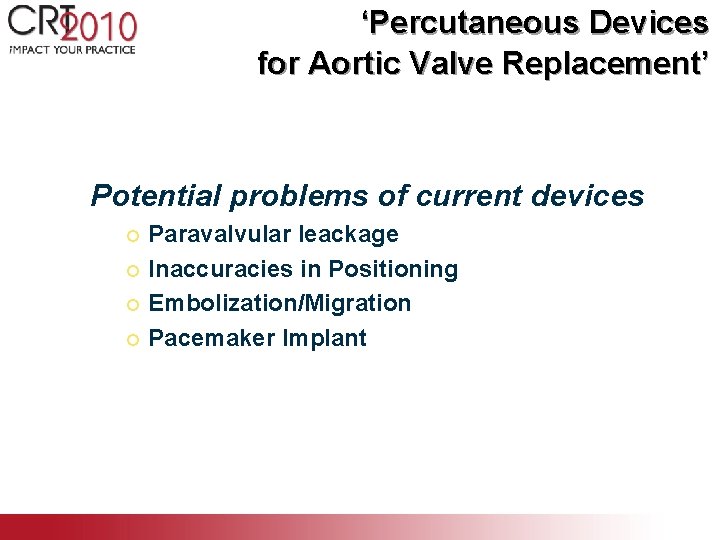
‘Percutaneous Devices for Aortic Valve Replacement’ Potential problems of current devices Paravalvular leackage ¡ Inaccuracies in Positioning ¡ Embolization/Migration ¡ Pacemaker Implant ¡

Future Aortic Valve Concepts ¡ Direct Flow ¡ Sadra ¡ Aor. Tx ¡ Jena Valve ¡ HLT ABPS Perc. Valve ¡ Endo. Tech ¡ Ventor Embracer ¡
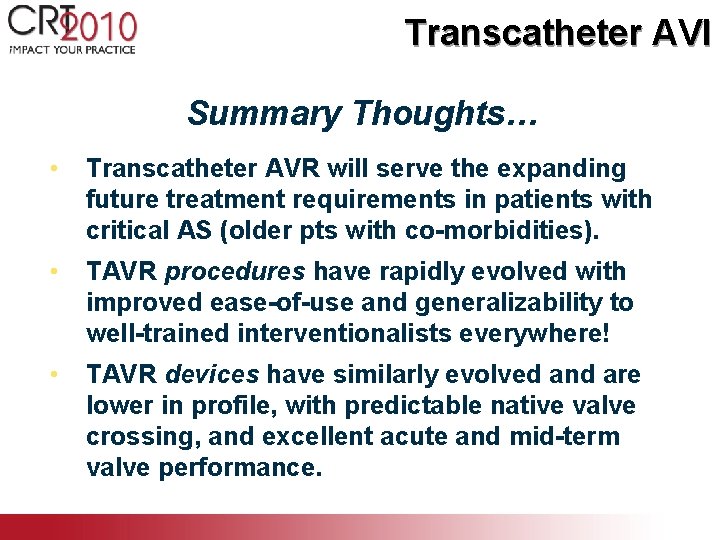
Transcatheter AVI Summary Thoughts… • Transcatheter AVR will serve the expanding future treatment requirements in patients with critical AS (older pts with co-morbidities). • TAVR procedures have rapidly evolved with improved ease-of-use and generalizability to well-trained interventionalists everywhere! • TAVR devices have similarly evolved and are lower in profile, with predictable native valve crossing, and excellent acute and mid-term valve performance.
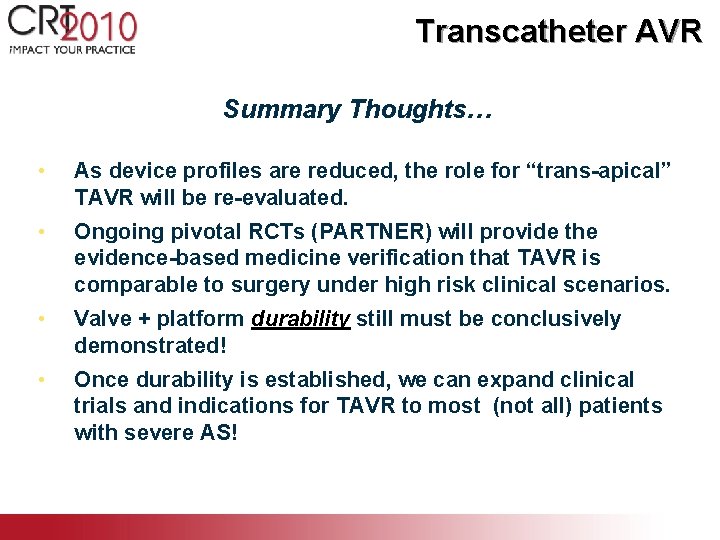
Transcatheter AVR Summary Thoughts… • As device profiles are reduced, the role for “trans-apical” TAVR will be re-evaluated. • Ongoing pivotal RCTs (PARTNER) will provide the evidence-based medicine verification that TAVR is comparable to surgery under high risk clinical scenarios. • Valve + platform durability still must be conclusively demonstrated! • Once durability is established, we can expand clinical trials and indications for TAVR to most (not all) patients with severe AS!
- Slides: 29