What molecules make up living things ORGANIC COMPOUNDS












- Slides: 42

What molecules make up living things? ORGANIC COMPOUNDS

What is an organic molecule? • Must contain the element _______ • Found in living organisms • Some organic molecules contain _____ and _____ • Some contain nitrogen (only_______)

What is an inorganic molecule? • Any molecule that is not organic is _____ • Does not contain ____ – Exception is ____ doesn’t contain H LIVING THINGS CONTAIN BOTH ORGANIC AND INORGANIC MOLECULES

4 Kinds of Organic Compounds • • __________ THESE MOLECULES CAN ALSO BE CALLED __________= BIG , MOLECULES= DIFFERENT

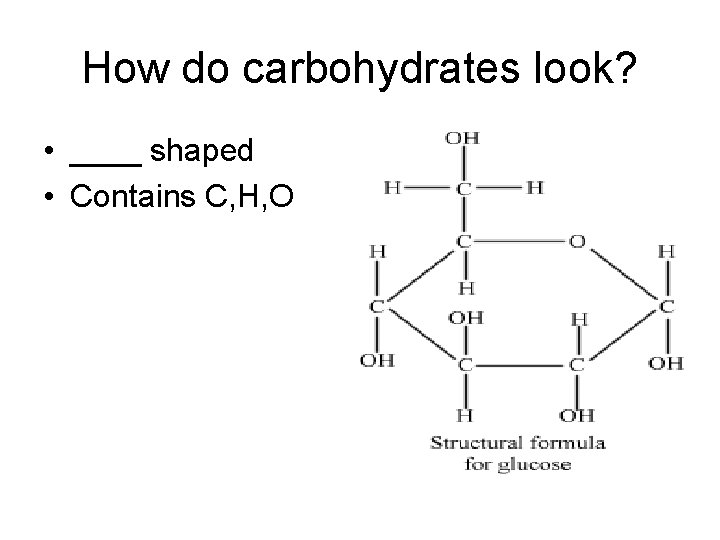
How do carbohydrates look? • ____ shaped • Contains C, H, O

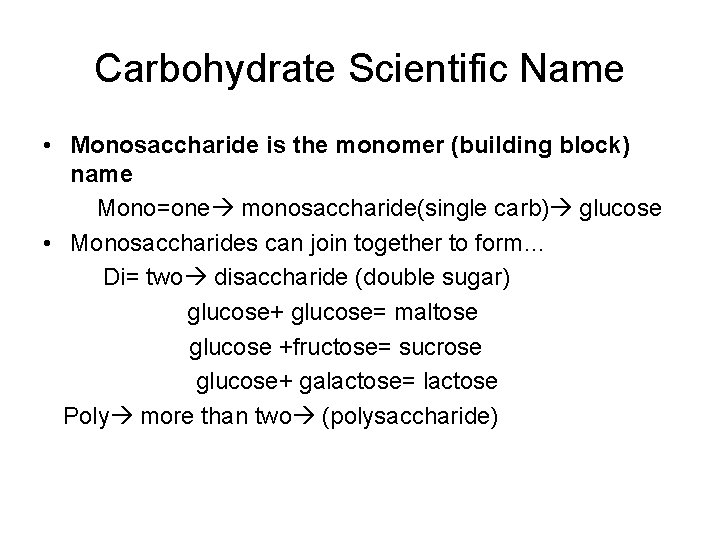
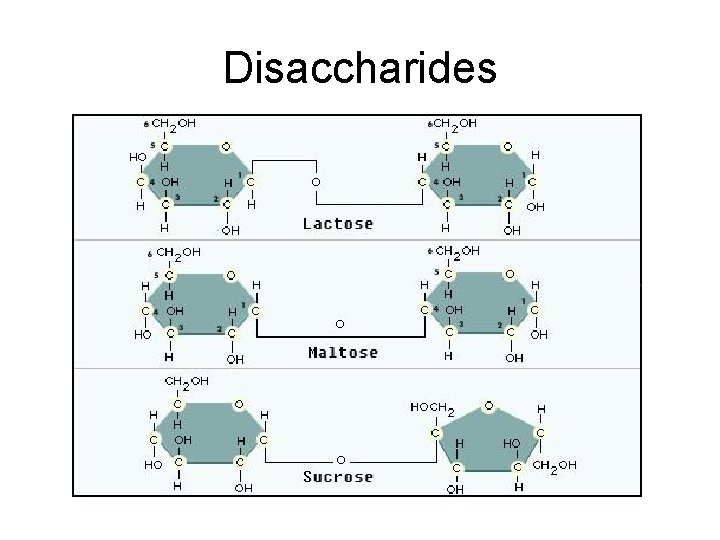
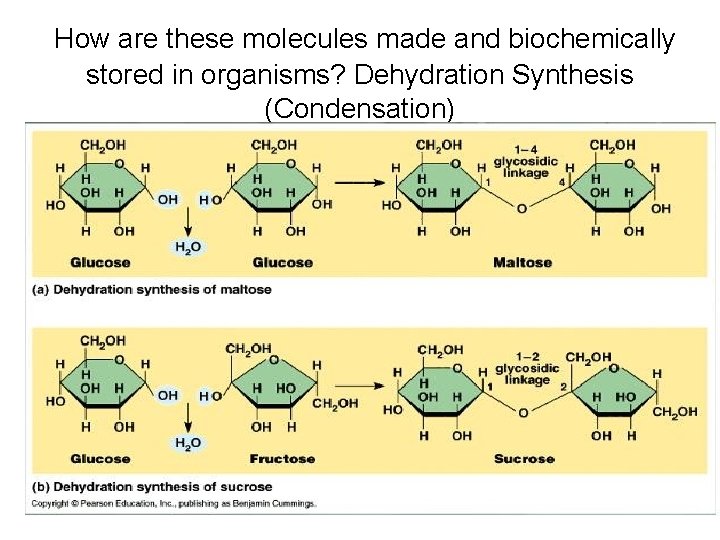
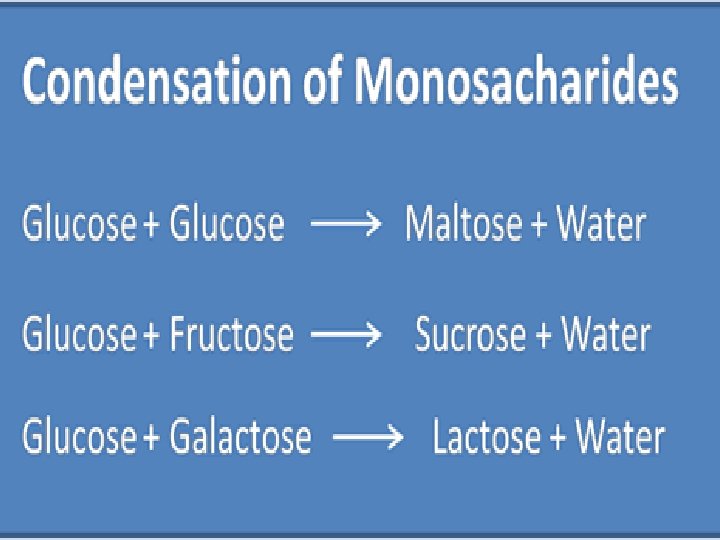
Carbohydrate Scientific Name • Monosaccharide is the monomer (building block) name Mono=one monosaccharide(single carb) glucose • Monosaccharides can join together to form… Di= two disaccharide (double sugar) glucose+ glucose= maltose glucose +fructose= sucrose glucose+ galactose= lactose Poly more than two (polysaccharide)

Examples of Carbohydrates • Monosaccharides – Glucose, fructose, galactose, ribose • Dissacharides – sucrose, lactose, maltose • Polysaccharides – Starch, cellulose, chitin, glycogen

Disaccharides

Polysaccharides

Carbohydrates- Biological Function and Features • Main source of usable ______ for organisms • Used in the presence of oxygen to generate cellular energy (ATP)= cellular respiration • Carbohydrates make up part of our cell membrane (hydrophobic) • Sweet in flavor – ______ is an important complex carbohydrate made from glucose – _____ is a carbohydrate that make up plant cell walls raw veggies are crunchy because you are eating the cell wall – We store carbohydrates in the liver in a form called GLYCOGEN

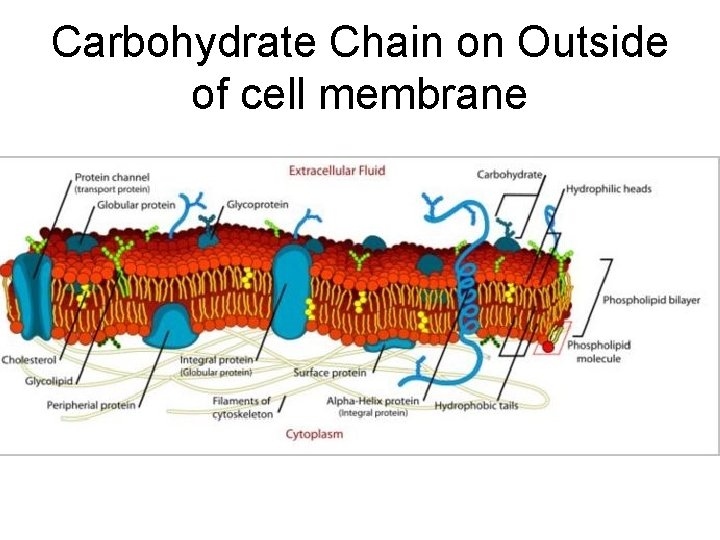
Carbohydrate Chain on Outside of cell membrane

How do living things obtain these carbohydrates? • Food that they eat – Grains and plants

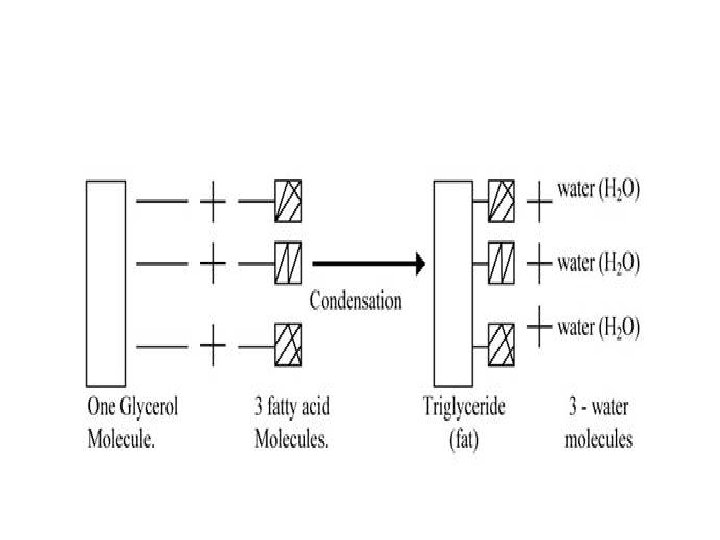
How are these molecules made and biochemically stored in organisms? Dehydration Synthesis (Condensation)

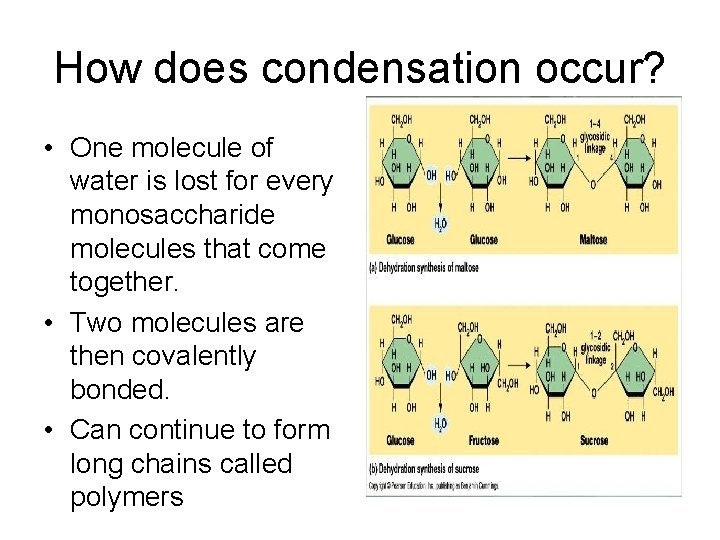
How does condensation occur? • One molecule of water is lost for every monosaccharide molecules that come together. • Two molecules are then covalently bonded. • Can continue to form long chains called polymers


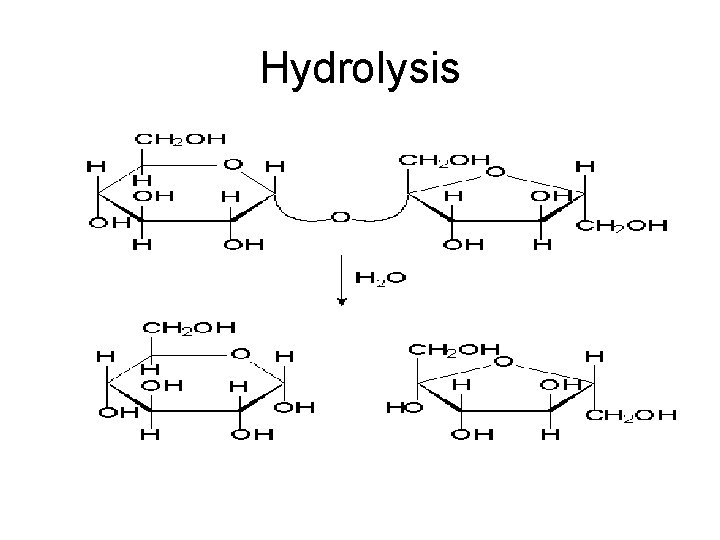
How do organisms break large carbohydrates for usage? • Hydrolysis • Using water to split di- and polysaccharides in order to form monosaccharides (glucose) • The monosaccharides can then be used by cell to generate cell energy (ATP)

Hydrolysis

Animation • http: //nhscience. lonestar. edu/biol/dehydrat. html

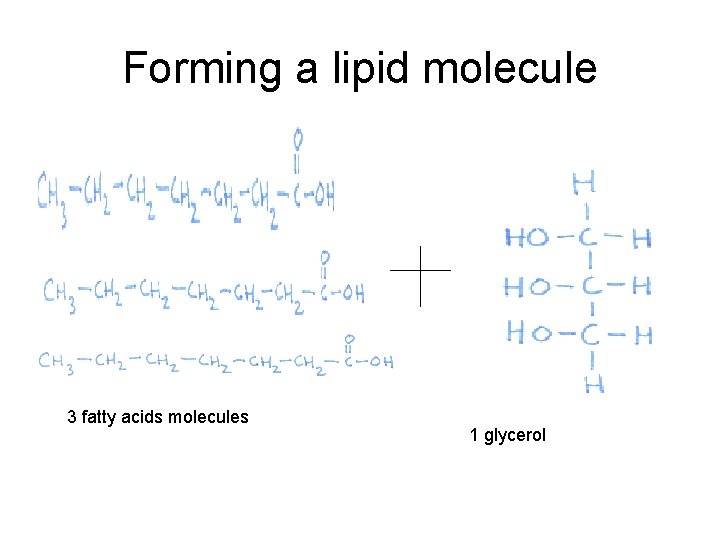
Lipids • Also known as ____ or ____ – Fat: solid at room temperature – Oil: liquid at room temp Monomer building blocks of two parts: Glycerol and 3 fatty acids

Forming a lipid molecule 3 fatty acids molecules 1 glycerol

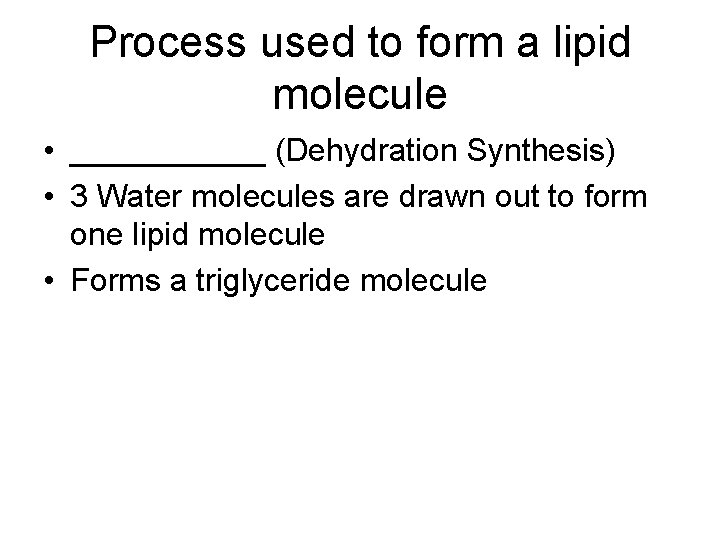
Process used to form a lipid molecule • ______ (Dehydration Synthesis) • 3 Water molecules are drawn out to form one lipid molecule • Forms a triglyceride molecule


Lipid formation animation • http: //nutrition. jbpub. com/resources/animat ions. cfm? id=10&debug=0

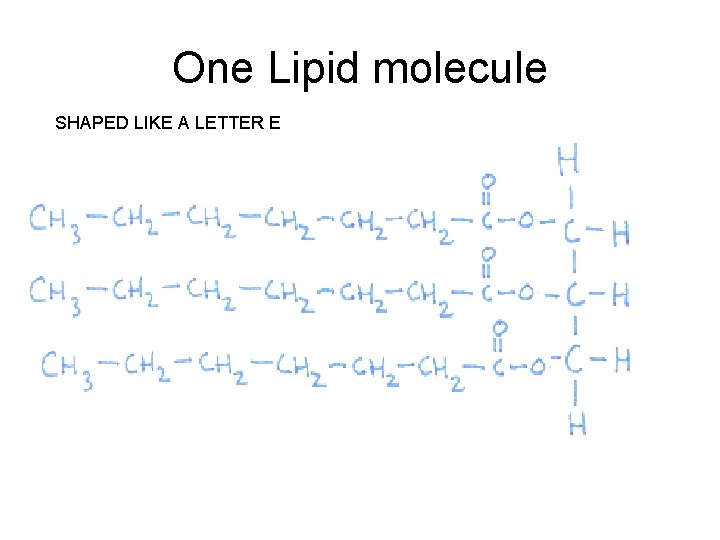
One Lipid molecule SHAPED LIKE A LETTER E

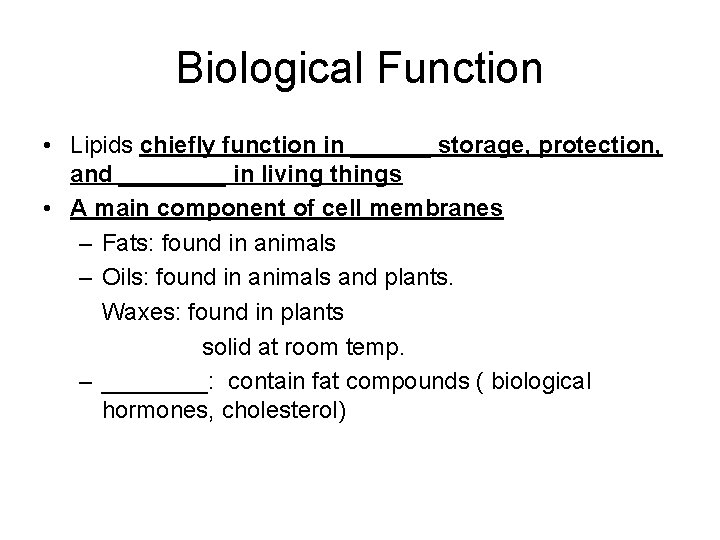
Biological Function • Lipids chiefly function in ______ storage, protection, and ____ in living things • A main component of cell membranes – Fats: found in animals – Oils: found in animals and plants. Waxes: found in plants solid at room temp. – ____: contain fat compounds ( biological hormones, cholesterol)

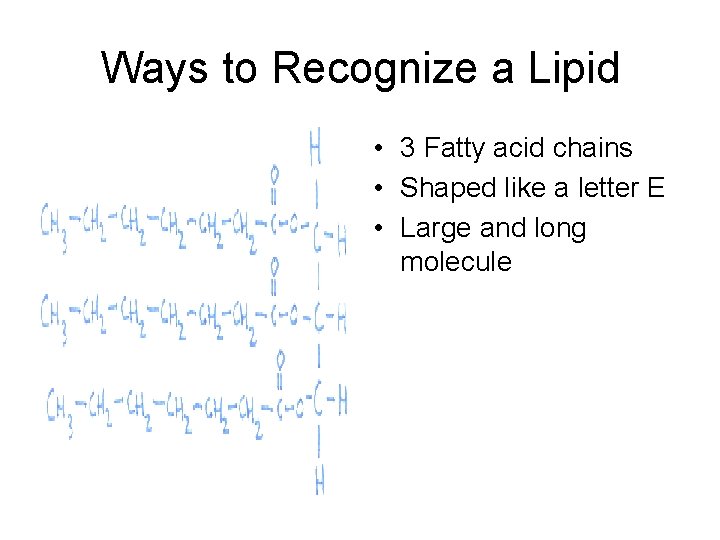
Ways to Recognize a Lipid • 3 Fatty acid chains • Shaped like a letter E • Large and long molecule

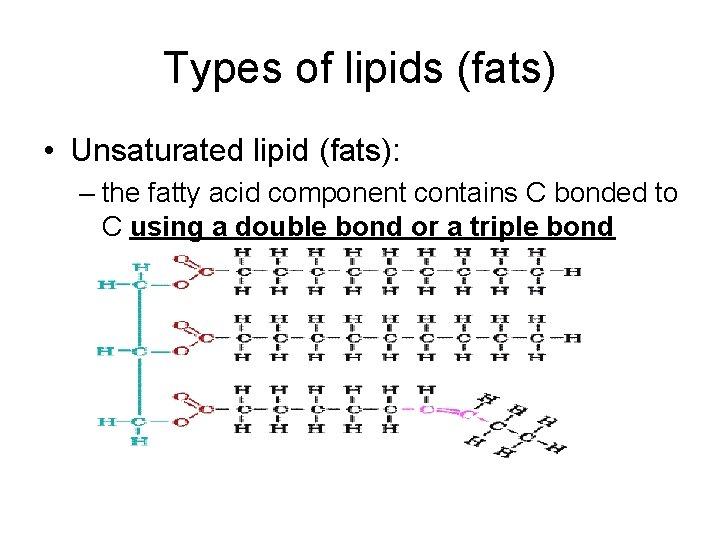
Types of lipids (fats) • Unsaturated lipid (fats): – the fatty acid component contains C bonded to C using a double bond or a triple bond

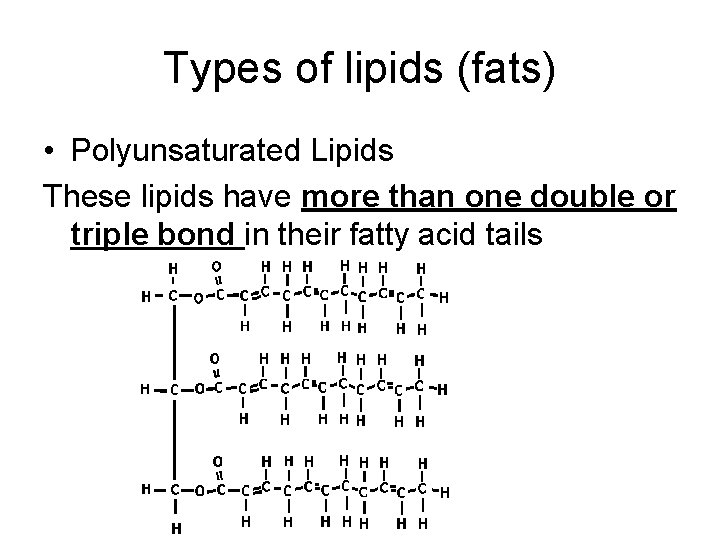
Types of lipids (fats) • Polyunsaturated Lipids These lipids have more than one double or triple bond in their fatty acid tails

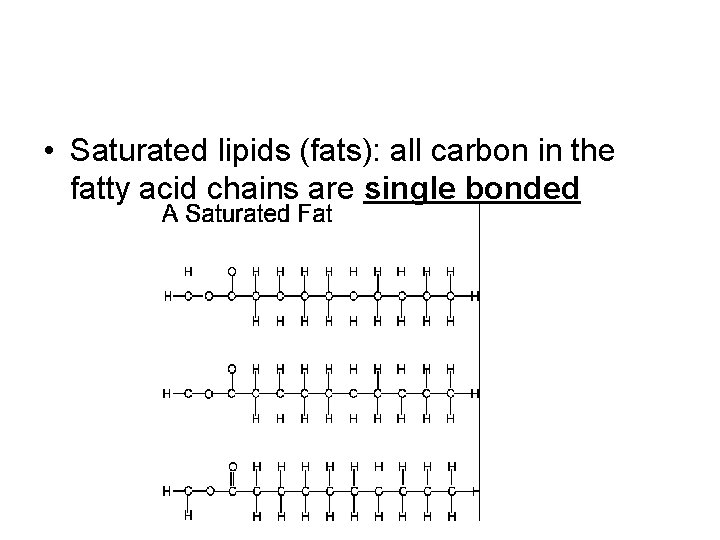
• Saturated lipids (fats): all carbon in the fatty acid chains are single bonded

What is a protein? • _______ are organic molecules that play an important role in • • _____ and _____ of cells Can be used for _____ Helps to keep a stable body temperature(_____) Growth and repair and support of muscle tissue, hair, skin, nails (ex. Keratin and collagen) • Carry out genetic _______ from the nucleus (in ___) • Helps to speed up biochemical reactions (_______) • Fighting off infections (antibodies)

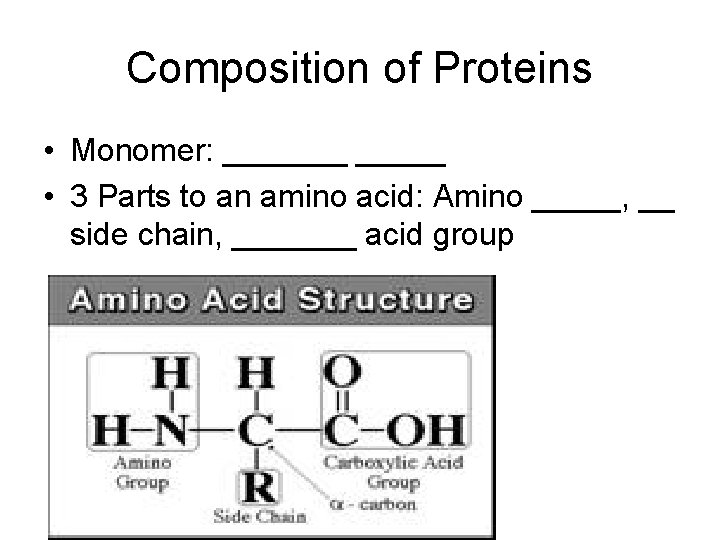
Composition of Proteins • Monomer: _______ • 3 Parts to an amino acid: Amino _____, __ side chain, _______ acid group

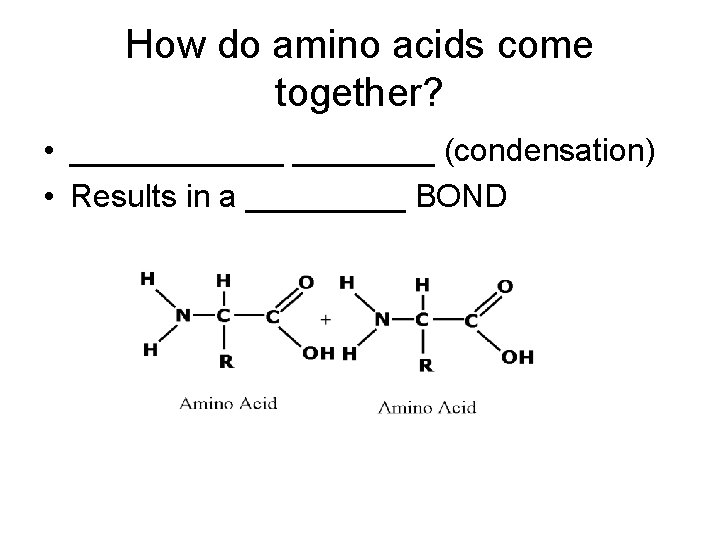
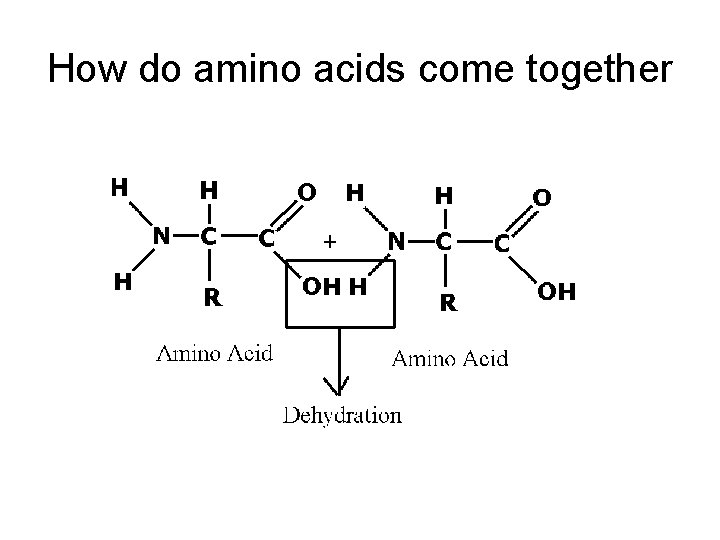
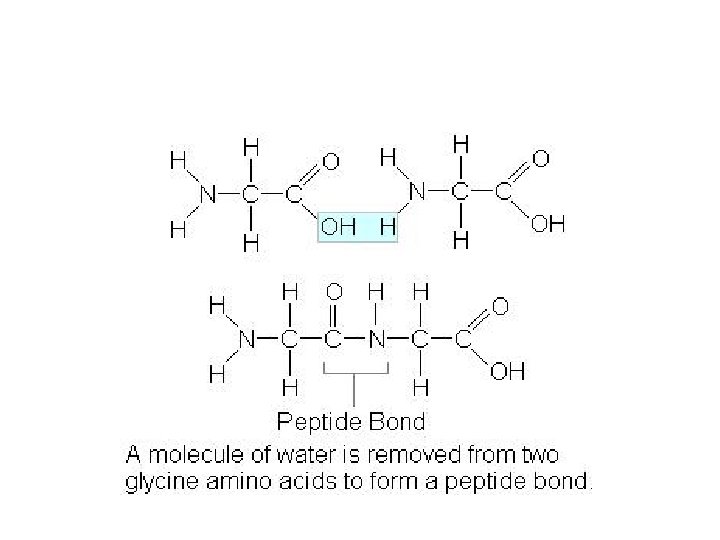
How do amino acids come together? • ______ (condensation) • Results in a _____ BOND

How do amino acids come together

How do amino acids form proteins? Condensation/ Dehydration Synthesis • Forms a _______ when amino acids combine • 2 a. a. coming together= __PEPTIDE • 3 or more a. a. coming together = ____PEPTIDE • 50 -3000 a. a. linked together considered a PROTEIN


Animation- Protein • http: //nhscience. lonestar. edu/biol/dehydrat. html

How can proteins change? • http: //www. sumanasinc. com/webcontent/a nimations/content/proteinstructure. html

What are nucleic acids? • Compounds that contain ____ and _____ in addition to other organic elements C, O, H • Found in ______ material in the form of ____ or ____

DNA- Deoxyribonucleic Acid • Contains the genetic hereditary code that makes each of us different. Our genetic “blueprint”

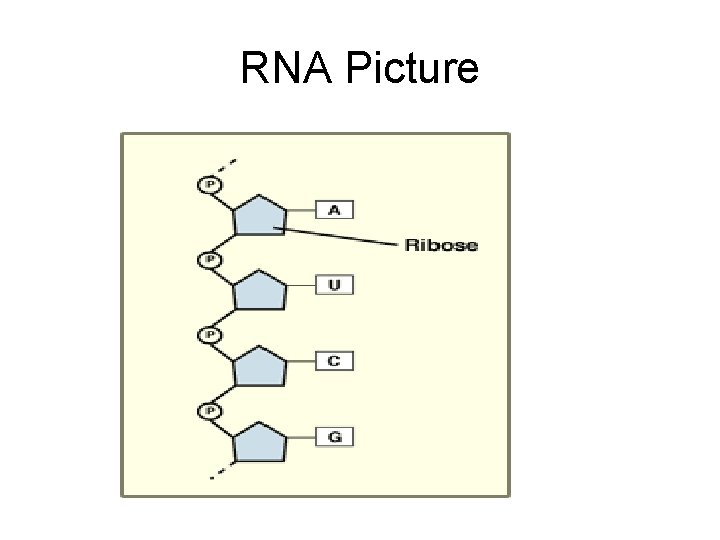
What is RNA? • RNA= _____ • RNA is _______ stranded • Controls genetic messages of the cell to form ____ for the cell. (takes place in ____)

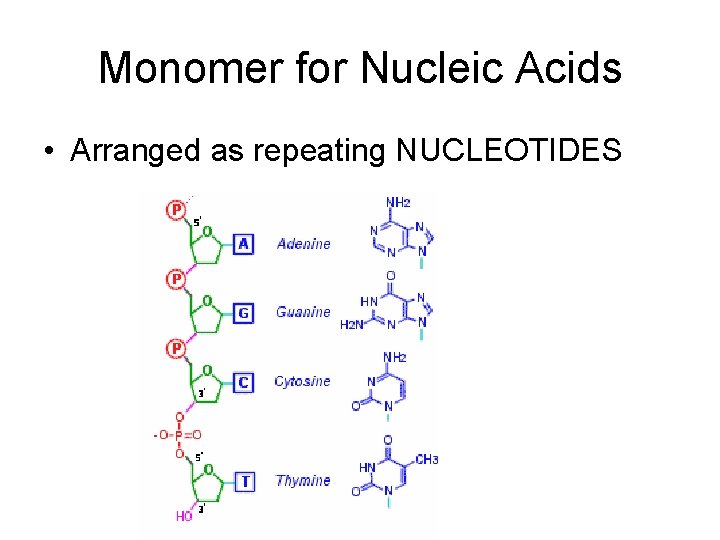
Monomer for Nucleic Acids • Arranged as repeating NUCLEOTIDES

RNA Picture