What is the relationship between atoms and elements
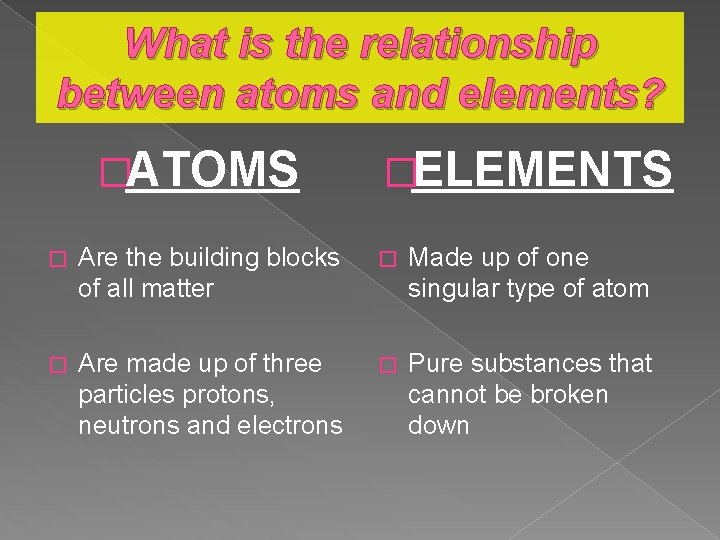
What is the relationship between atoms and elements? �ATOMS �ELEMENTS � Are the building blocks of all matter � Made up of one singular type of atom � Are made up of three particles protons, neutrons and electrons � Pure substances that cannot be broken down

WELCOME TO THE MAIN EVENT!! Your Name ATOMS 8. P. 1. 2. – PART 1 • Bell # What is an atom? What is a proton, neutron and an electron? How do electrons combine to make molecules and compounds?

What is the smallest Unit and building block of all matter? http: //www. youtube. com/watch? v=cn. XV 7 Ph 3 WPk Let’s watch a short clip about atoms and how they work!
What is the smallest piece and the building block of all matter? The Smallest Piece of Matter: The Atom

ALUMINUM! If we keep breaking down this aluminum foil into smaller and smaller pieces, when would we ever stop? What do you think is the smallest piece of aluminum foil that exists? Would you be able to see it? How?

What about this copper penny? Is it any different than aluminum foil at that smallest level?

The smallest piece of aluminum is an aluminum atom, and the smallest piece of copper is a copper atom.

For this lesson, our focus is going to be on atoms and elements. By the end of this lesson, you should be able to answer the following questions: • What is the relationship between an atom and an element? • How are protons and neutrons different from and similar to each other? • How do electrons differ from both protons and neutrons?

Lets finish watching: The Smallest Piece of Matter: The Atom You will learn what an atom is, and that different types of matter are made of different types of atoms.

Which are made o f atoms? Cans Fog Chief Keef Wood Water Your hair Animals Light Carrie Underwood Heat

Atoms • Definition- Atoms are the building blocks of all matter (sort of how bricks are the building blocks of houses) • The smallest amount of matter you can have is ONE ATOM.

Atoms and Elements! http: //www. bbc. co. uk/bitesize/ks 3/science/chemical_material_behaviour/ atoms_elements/activity/
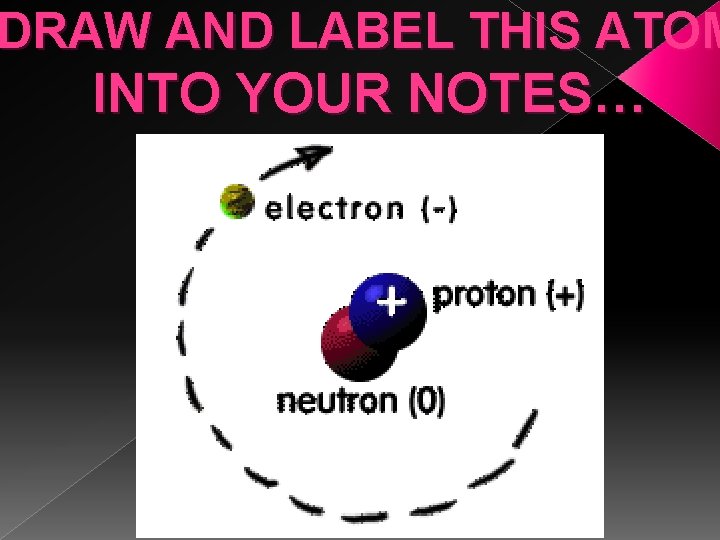
DRAW AND LABEL THIS ATOM INTO YOUR NOTES…

• What kinds of atoms are in aluminum foil? • What kinds of atoms are in copper wire? • How are atoms of aluminum and copper atoms different from each other? Try to answer these questions while watching the video: Elements (0: 08– 1: 21)
Let’s watch: Introduction to Atomic Structure Can you tell me what makes one atom different than another atom?
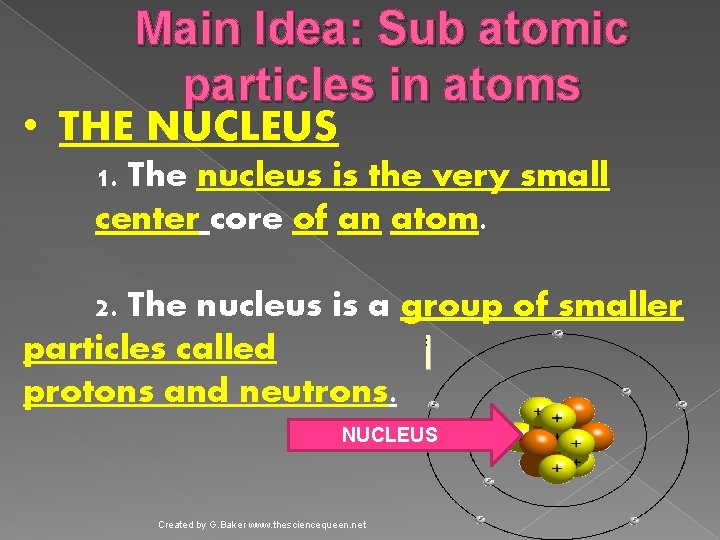
Main Idea: Sub atomic particles in atoms • THE NUCLEUS 1. The nucleus is the very small center core of an atom. 2. The nucleus is a group of smaller particles called protons and neutrons. NUCLEUS Created by G. Baker www. thesciencequeen. net
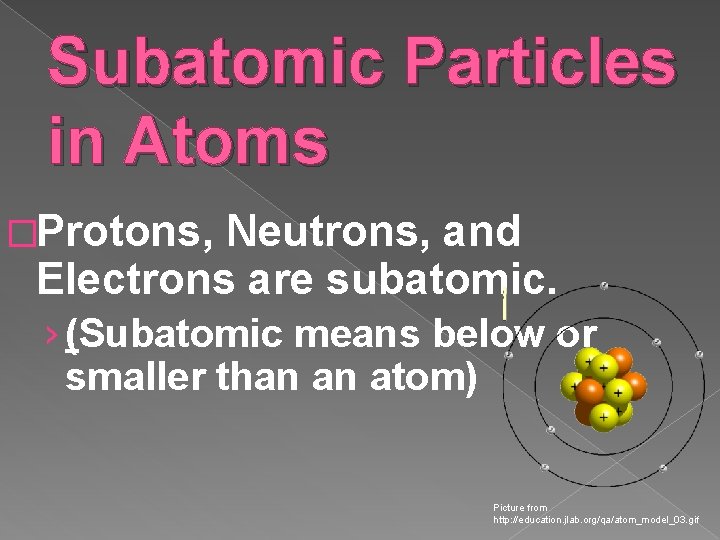
Subatomic Particles in Atoms �Protons, Neutrons, and Electrons are subatomic. › (Subatomic means below or smaller than an atom) Picture from http: //education. jlab. org/qa/atom_model_03. gif
Brain Break! http: //www. youtube. com/watch? v=O 5 iaw 5 WNu. B 0
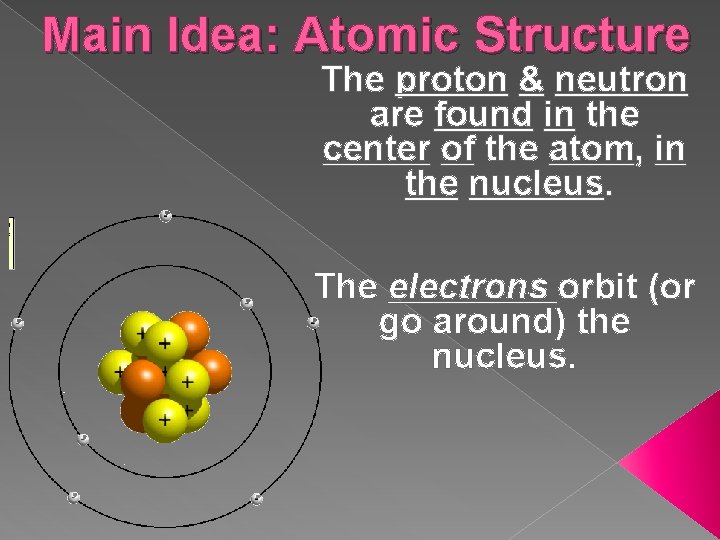
Main Idea: Atomic Structure The proton & neutron are found in the center of the atom, in the nucleus. The electrons orbit (or go around) the nucleus.
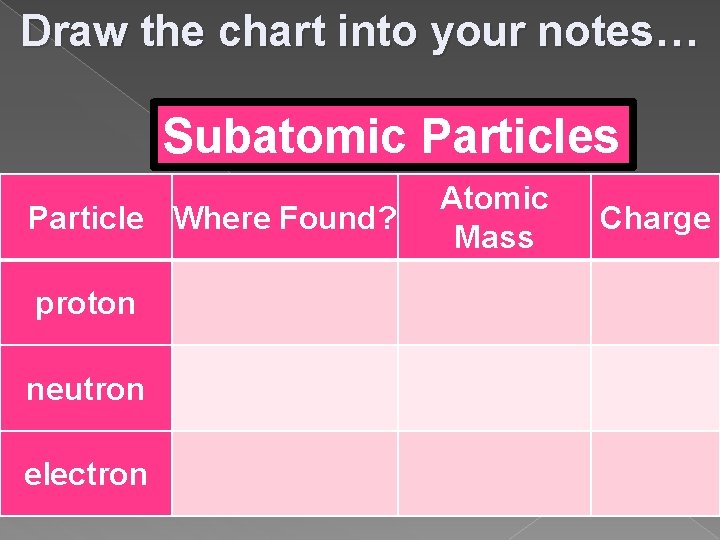
Draw the chart into your notes… Subatomic Particles Particle Where Found? Atomic Mass Charge proton neutron electron
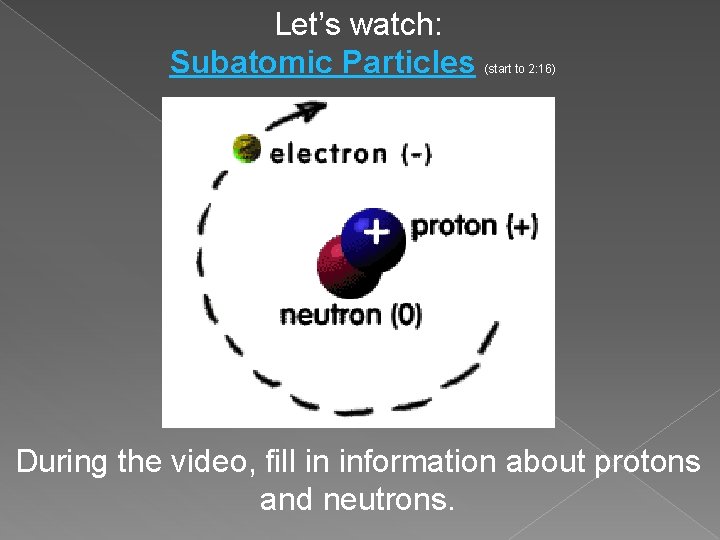
Let’s watch: Subatomic Particles (start to 2: 16) During the video, fill in information about protons and neutrons.

What is atomic mass? Atomic mass is the mass or the weight of an atom of a chemical element # Protons + # Neutrons = Atomic Mass
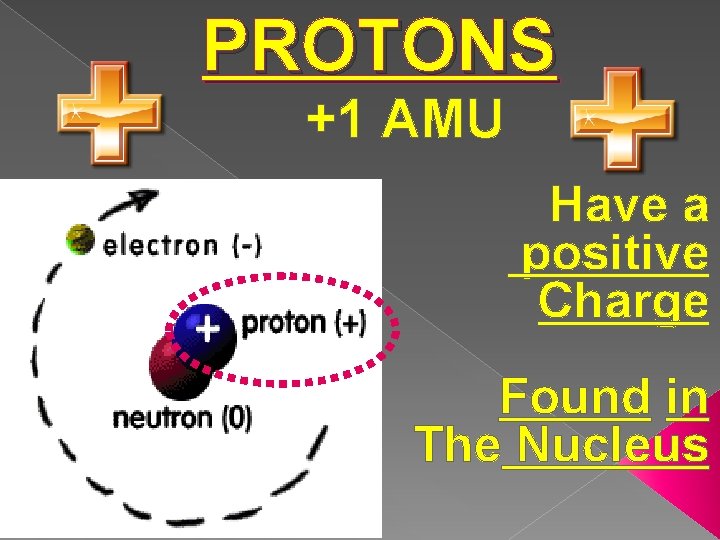
PROTONS +1 AMU Have a positive Charge Found in The Nucleus
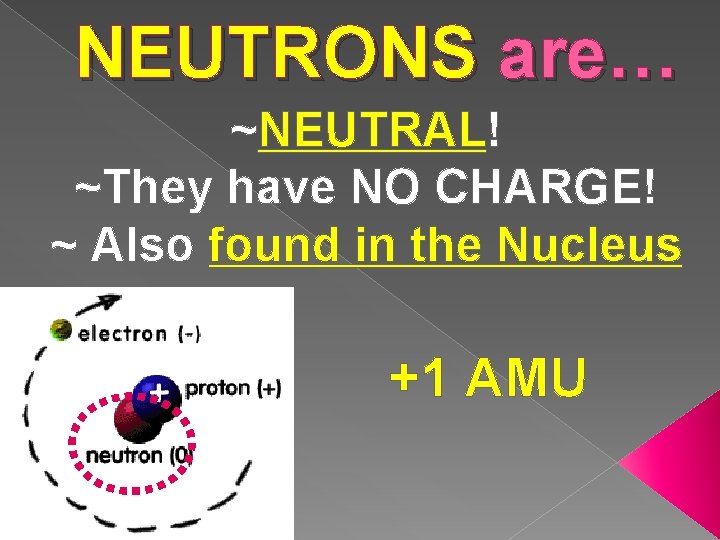
NEUTRONS are… ~NEUTRAL! ~They have NO CHARGE! ~ Also found in the Nucleus +1 AMU
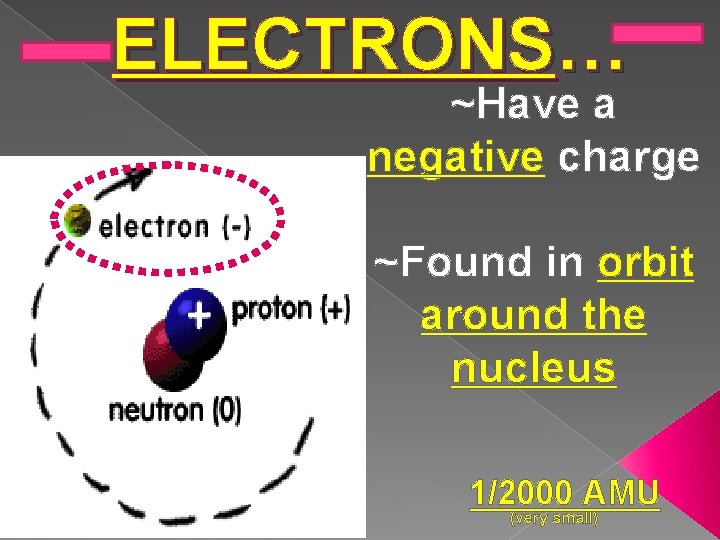
ELECTRONS… ~Have a negative charge ~Found in orbit around the nucleus 1/2000 AMU (very small)
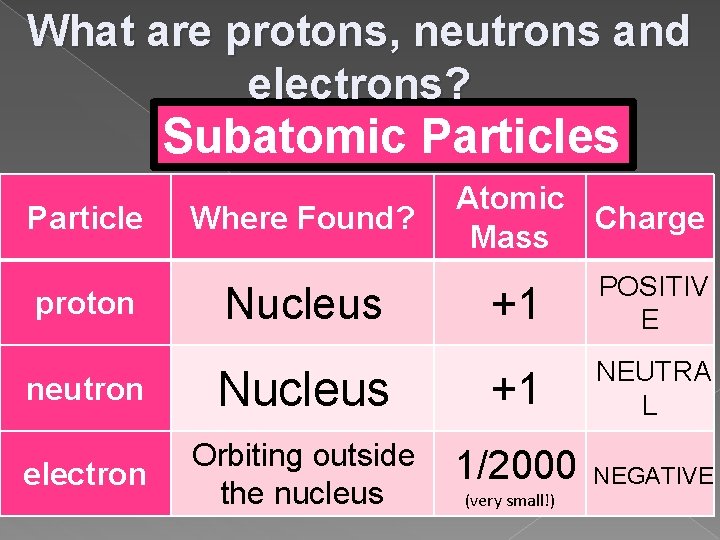
What are protons, neutrons and electrons? Subatomic Particles Particle Where Found? Nucleus proton Nucleus neutron electron Orbiting outside the nucleus Atomic Charge Mass +1 POSITIV E +1 NEUTRA L 1/2000 NEGATIVE (very small!)
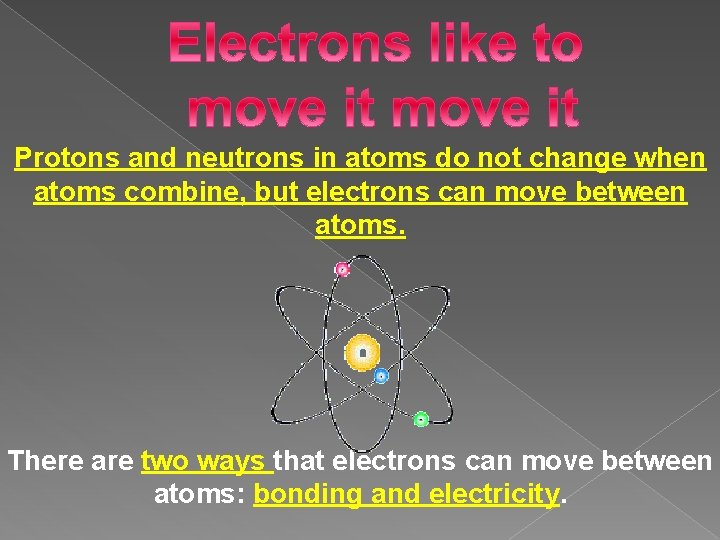
Protons and neutrons in atoms do not change when atoms combine, but electrons can move between atoms. There are two ways that electrons can move between atoms: bonding and electricity.
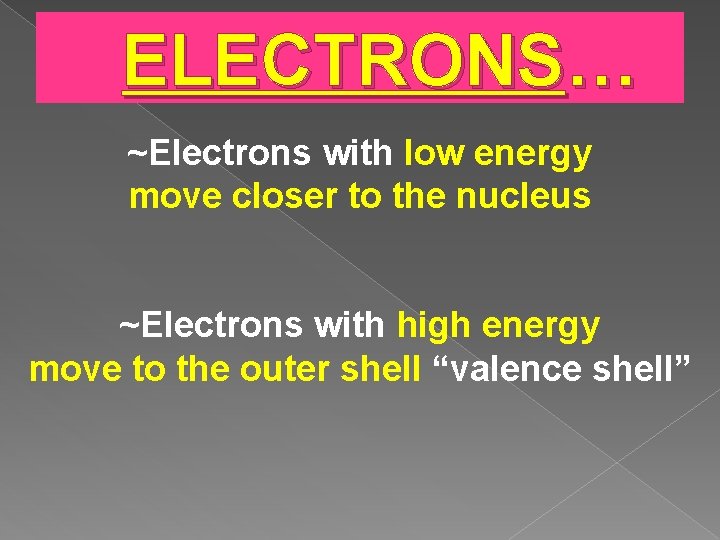
ELECTRONS… ~Electrons with low energy move closer to the nucleus ~Electrons with high energy move to the outer shell “valence shell”

Let’s take an even closer look… Subatomic Particles (1: 35 -2: 15) Take notes on the information shown about carbon.
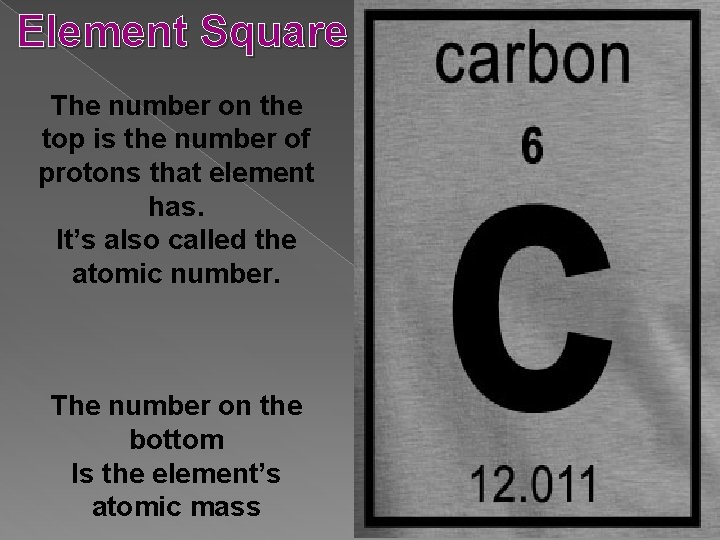
Element Square The number on the top is the number of protons that element has. It’s also called the atomic number. The number on the bottom Is the element’s atomic mass


How many protons are in an atom of lithium? How many protons are in an atom of bromine? How many protons are in an atom of iron?

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? • What is the relationship between an atom and an element? • How are protons and neutrons different from and similar to each other? • How do electrons differ from both protons and neutrons? • How are protons, neutrons, and electrons arranged to form an atom? • What are two ways electrons can be gained or lost from an atom?

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? What is the relationship between an atom and an element? An atom is a fundamental building block of matter, and an element is a pure substance that’s made of just one kind of atom.

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? How are protons and neutrons different from and similar to each other? Protons have a positive charge, while neutrons are neutral. Both protons and neutrons are located in the nucleus of an atom. Both protons and neutrons have similar mass.

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? How do electrons differ from both protons and neutrons? Electrons are found in the relatively large region of an atom outside the nucleus. Electrons are negatively charged and have a much smaller mass than protons and neutrons.

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? How are protons, neutrons, and electrons arranged to form an atom? Both protons and neutrons are located in the nucleus of an atom. Electrons are found in the larger region of an atom outside the nucleus.

CAN WE ANSWER OUR ESSENTIAL QUESTIONS? What are two ways electrons can be gained or lost from an atom? Electrons can be gained or lost from atoms 1) as bonds are formed or broken between atoms, or 2) through movements as part of an electric current.

- Slides: 39