What is the formulae 1 2 3 4





- Slides: 25

What is the formulae? 1. 2. 3. 4. 5. Hydrochloric acid Sulfuric acid Sodium hydroxide Sodium chloride Sodium sulfate

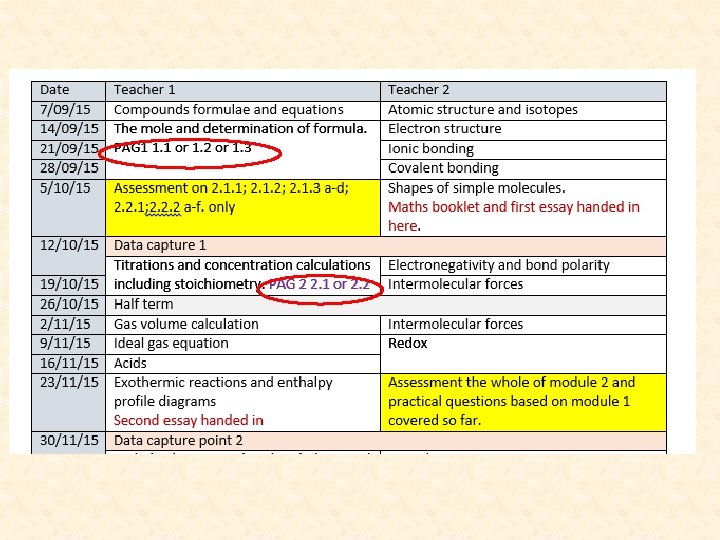
1. 2. 3. 4. 5. Starter: what is the formulae? Hydrochloric acid HCl Sulfuric acid H 2 SO 4 Sodium hydroxide Na. OH Sodium chloride Na. Cl Sodium sulfate Na 2 SO 4

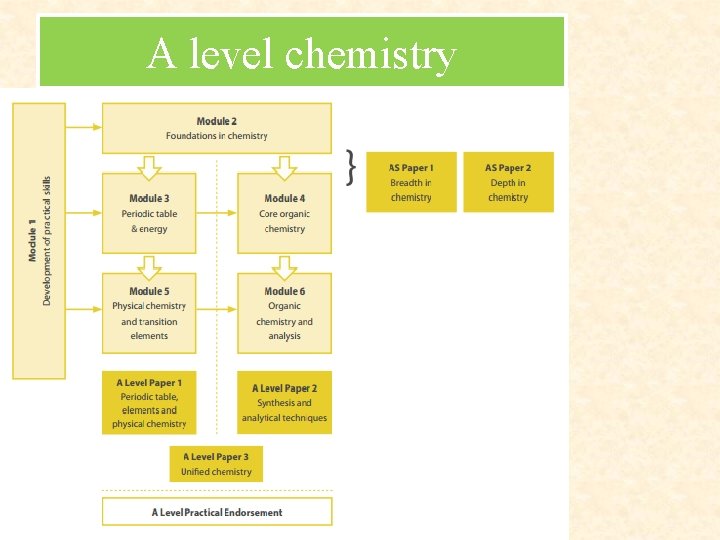
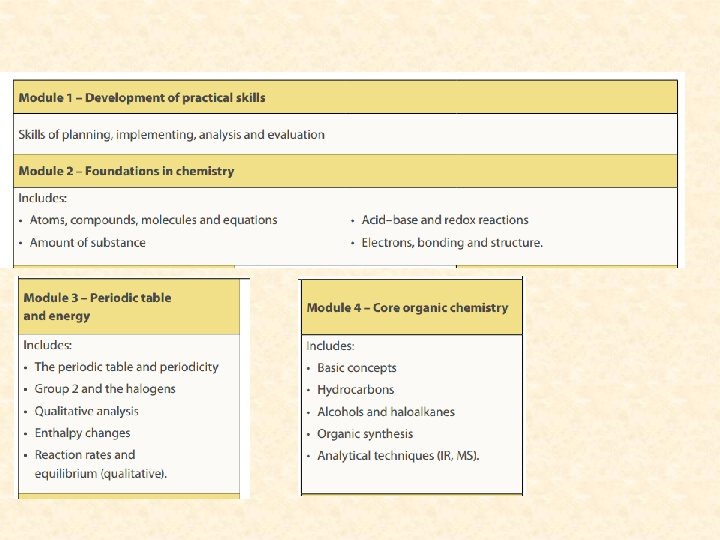
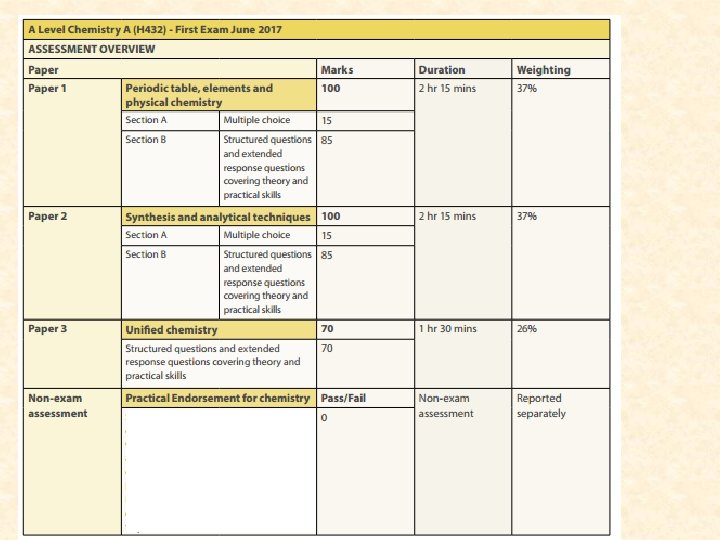
A level chemistry



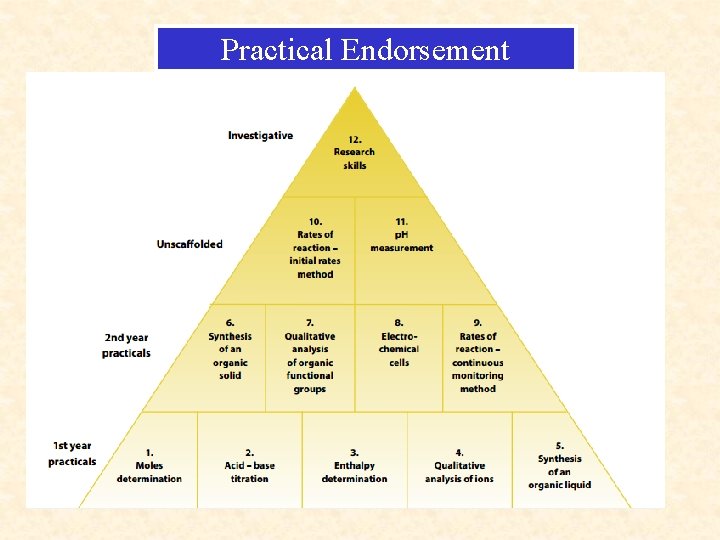
Practical Endorsement



Text books We lend you this one Highly recommended, especially if not a whizz at maths One of the many revision guides available.

Any questions?

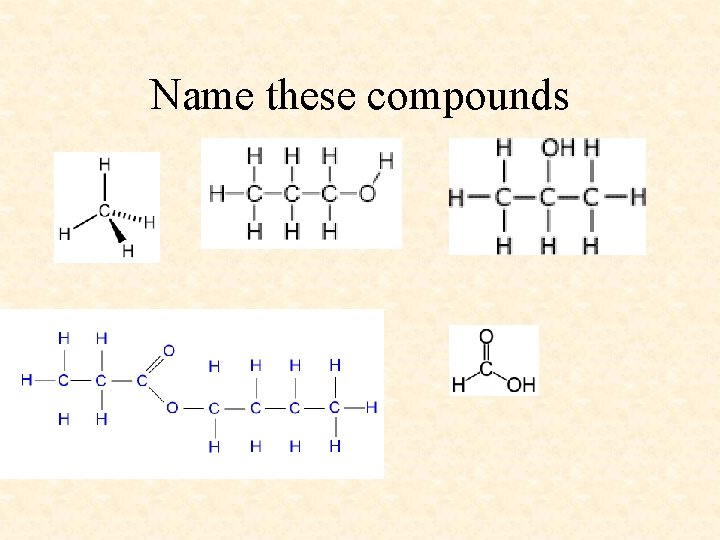
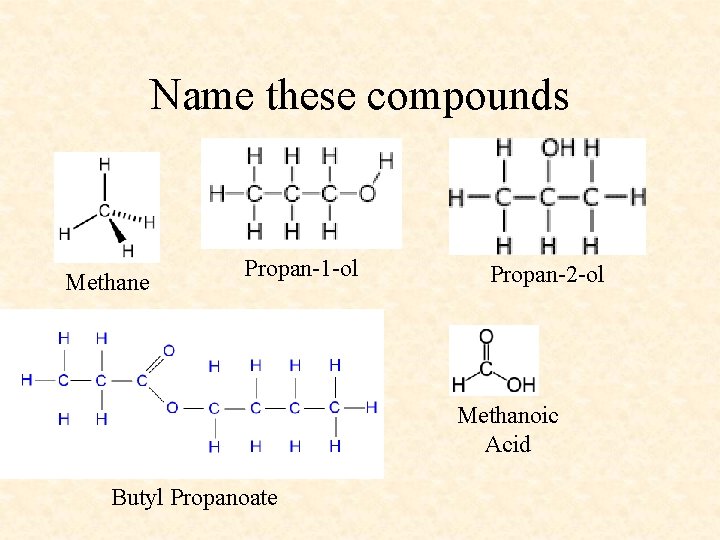
Name these compounds

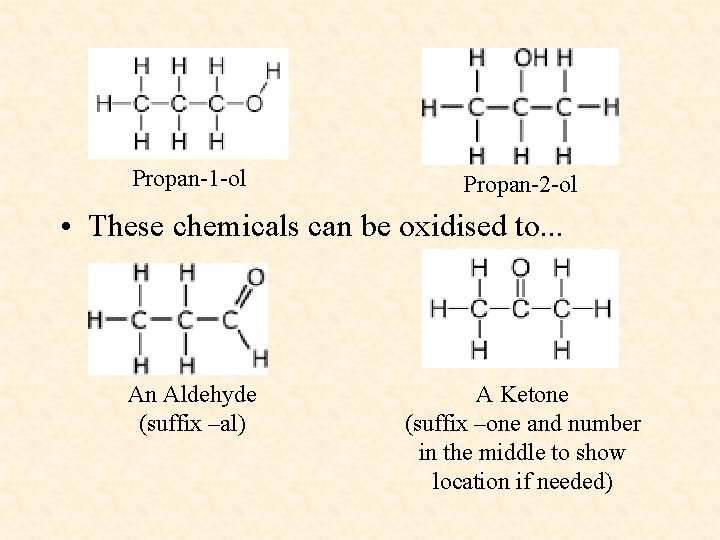
Name these compounds Methane Propan-1 -ol Propan-2 -ol Methanoic Acid Butyl Propanoate

Propan-1 -ol Propan-2 -ol • These chemicals can be oxidised to. . . An Aldehyde (suffix –al) A Ketone (suffix –one and number in the middle to show location if needed)

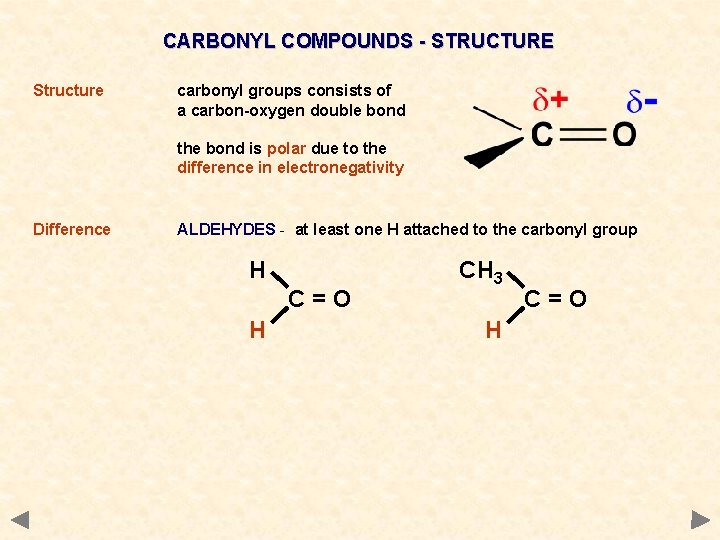
CARBONYL COMPOUNDS - STRUCTURE Structure carbonyl groups consists of a carbon-oxygen double bond the bond is polar due to the difference in electronegativity Difference ALDEHYDES - at least one H attached to the carbonyl group H C=O H CH 3 H C=O

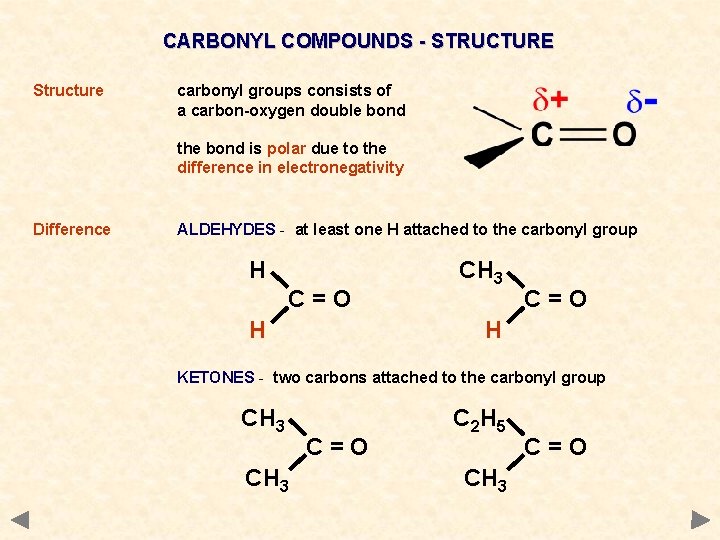
CARBONYL COMPOUNDS - STRUCTURE Structure carbonyl groups consists of a carbon-oxygen double bond the bond is polar due to the difference in electronegativity Difference ALDEHYDES - at least one H attached to the carbonyl group H C=O CH 3 C=O H H KETONES - two carbons attached to the carbonyl group CH 3 C=O C 2 H 5 CH 3 C=O

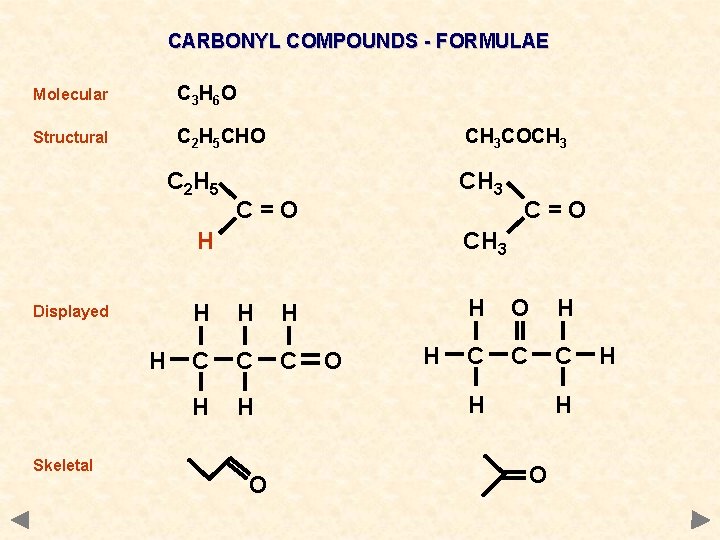
CARBONYL COMPOUNDS - FORMULAE Molecular C 3 H 6 O Structural C 2 H 5 CHO C 2 H 5 CH 3 COCH 3 C=O H Displayed H Skeletal C=O CH 3 H H H C C C H H O O H H O H C C C H H O H

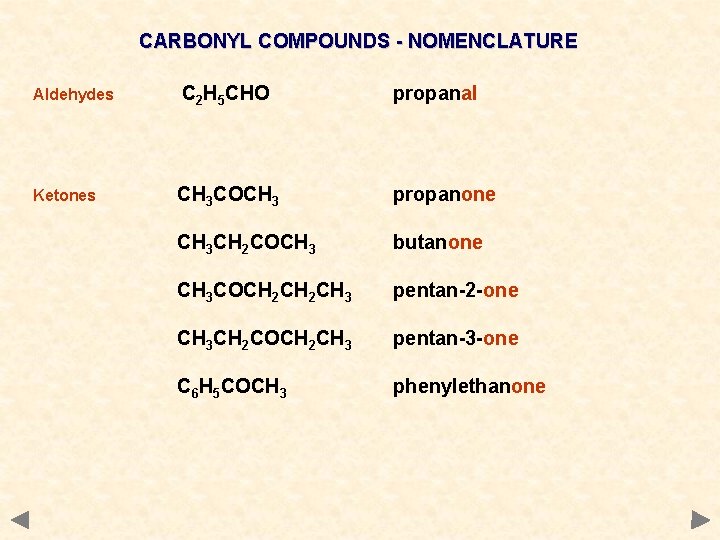
CARBONYL COMPOUNDS - NOMENCLATURE Aldehydes C 2 H 5 CHO propanal Ketones CH 3 COCH 3 propanone CH 3 CH 2 COCH 3 butanone CH 3 COCH 2 CH 3 pentan-2 -one CH 3 CH 2 COCH 2 CH 3 pentan-3 -one C 6 H 5 COCH 3 phenylethanone

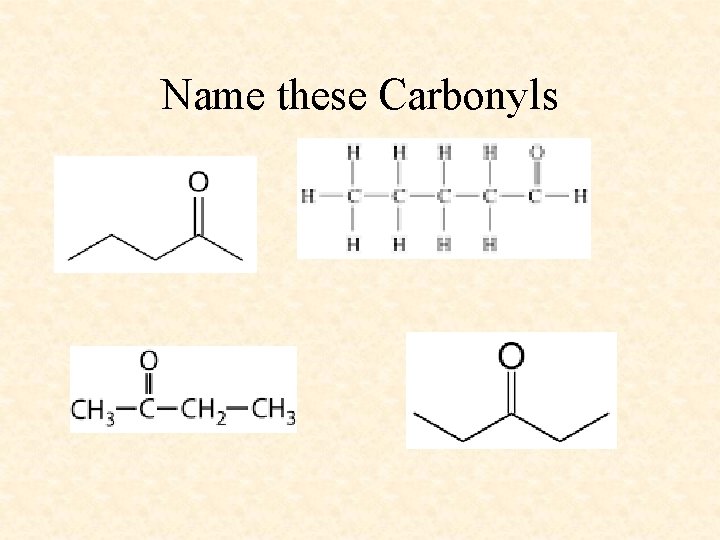
Name these Carbonyls

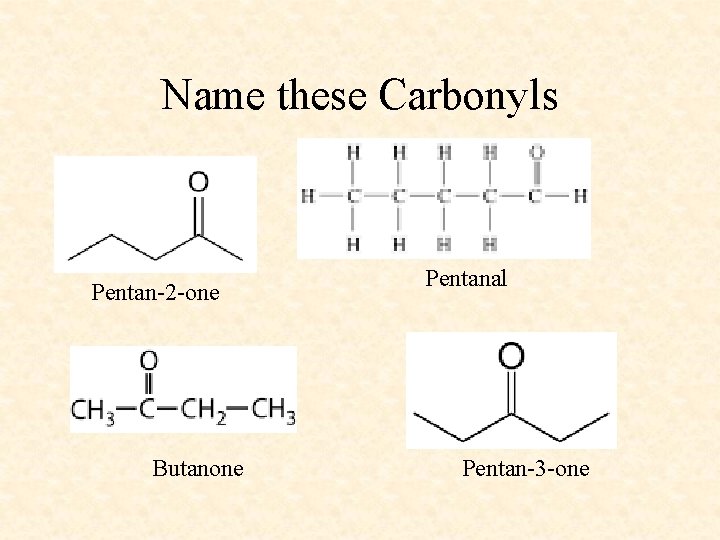
Name these Carbonyls Pentan-2 -one Butanone Pentanal Pentan-3 -one

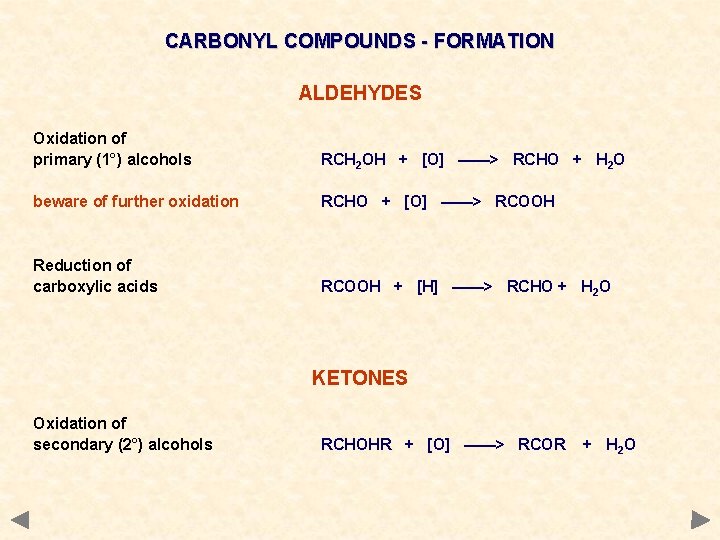
CARBONYL COMPOUNDS - FORMATION ALDEHYDES Oxidation of primary (1°) alcohols RCH 2 OH + [O] ——> RCHO + H 2 O beware of further oxidation RCHO + [O] ——> RCOOH Reduction of carboxylic acids RCOOH + [H] ——> RCHO + H 2 O KETONES Oxidation of secondary (2°) alcohols RCHOHR + [O] ——> RCOR + H 2 O

Practical Skills 1) Prepare your workstation for safe working. 2) Prepare some Tollens Reagent by placing 1 cm 3 of Silver Nitrate solution into a test tube. Add one drop of Sodium Hydroxide Solution to the Silver Nitrate solution. 3) Dissolve the precipitate formed by adding Ammonia solution drop-wise until the precipitate JUST dissolves. 4) Divide the solution into 2 by pouring into 2 clean test tubes. 5) Add 10 drops of propanal to one of the tubes and 10 drops of propanone to the other. Label the tubes. 6) Place both tubes into a beaker of boiled water. Leave until no further changes occur (approx 5 min). Record your observations. 7) Put 1 cm 3 of acidified Potassium Dichromate (VI) solution into a clean test tube and add 10 drops of propanal. Warm Gently 8) Repeat step 7 using propanone instead of propanal and record your observations.

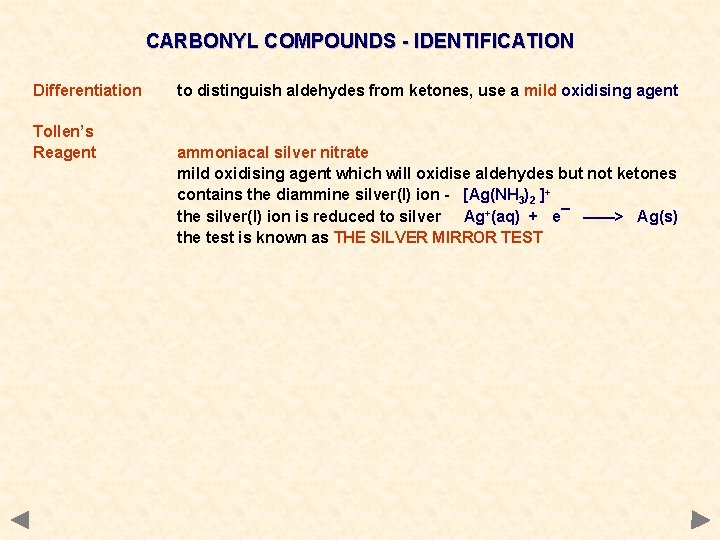
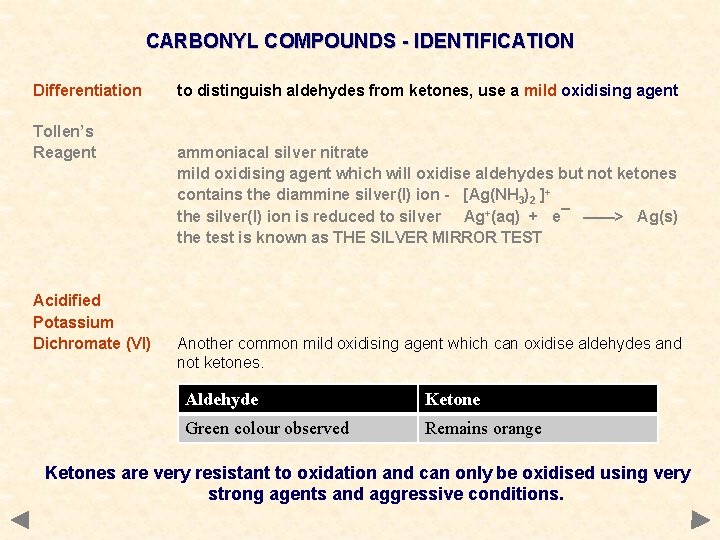
CARBONYL COMPOUNDS - IDENTIFICATION Differentiation Tollen’s Reagent to distinguish aldehydes from ketones, use a mild oxidising agent ammoniacal silver nitrate mild oxidising agent which will oxidise aldehydes but not ketones contains the diammine silver(I) ion - [Ag(NH 3)2 ]+ the silver(I) ion is reduced to silver Ag+(aq) + e¯ ——> Ag(s) the test is known as THE SILVER MIRROR TEST

CARBONYL COMPOUNDS - IDENTIFICATION Differentiation Tollen’s Reagent Acidified Potassium Dichromate (VI) to distinguish aldehydes from ketones, use a mild oxidising agent ammoniacal silver nitrate mild oxidising agent which will oxidise aldehydes but not ketones contains the diammine silver(I) ion - [Ag(NH 3)2 ]+ the silver(I) ion is reduced to silver Ag+(aq) + e¯ ——> Ag(s) the test is known as THE SILVER MIRROR TEST Another common mild oxidising agent which can oxidise aldehydes and not ketones. Aldehyde Ketone Green colour observed Remains orange Ketones are very resistant to oxidation and can only be oxidised using very strong agents and aggressive conditions.

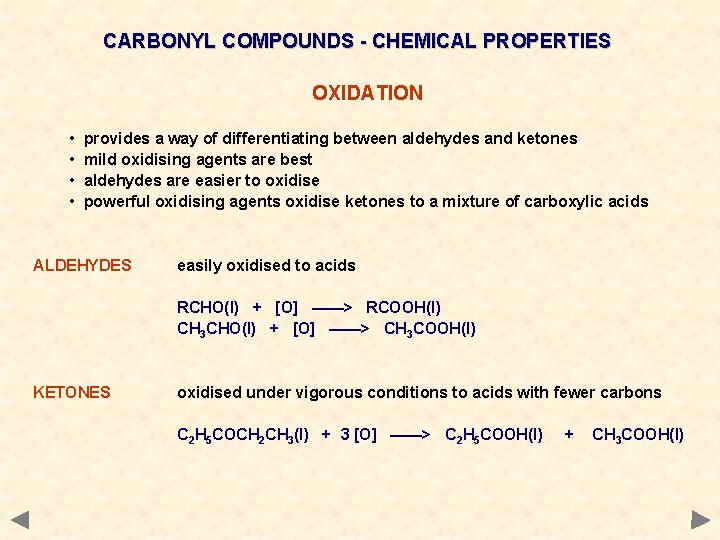
CARBONYL COMPOUNDS - CHEMICAL PROPERTIES OXIDATION • • provides a way of differentiating between aldehydes and ketones mild oxidising agents are best aldehydes are easier to oxidise powerful oxidising agents oxidise ketones to a mixture of carboxylic acids ALDEHYDES easily oxidised to acids RCHO(l) + [O] ——> RCOOH(l) CH 3 CHO(l) + [O] ——> CH 3 COOH(l) KETONES oxidised under vigorous conditions to acids with fewer carbons C 2 H 5 COCH 2 CH 3(l) + 3 [O] ——> C 2 H 5 COOH(l) + CH 3 COOH(l)

Hope you have enjoyed this brief taster. Any Questions?